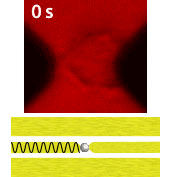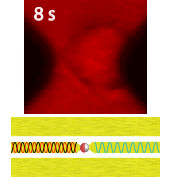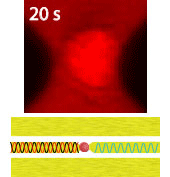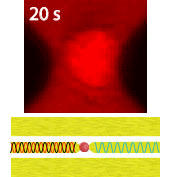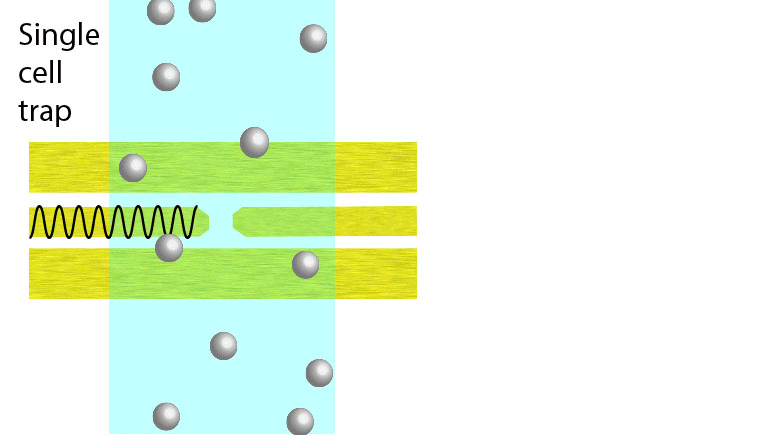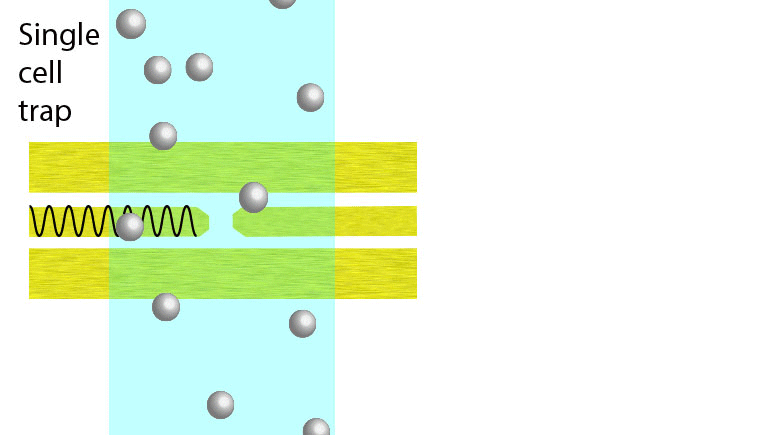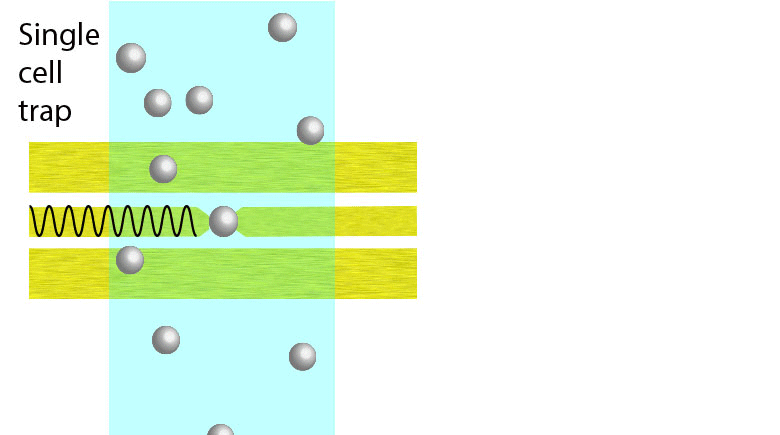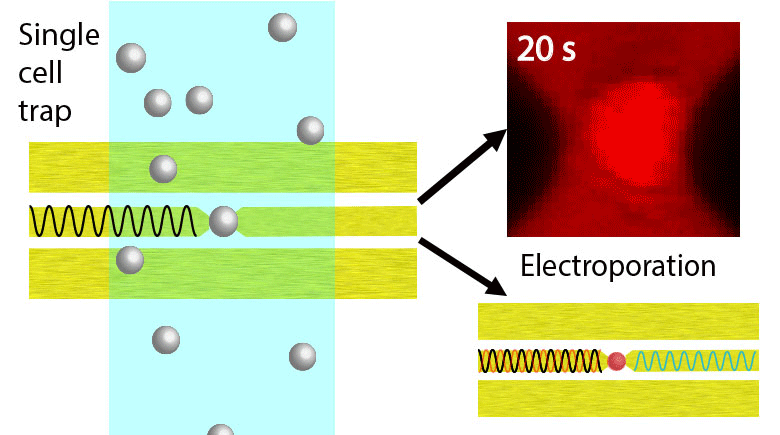
Electroporation is a widely used physical (as opposed to chemical or biological) method to enhance the permeability of a cell membrane, with the degree of enhancement determined by applied field magnitude, duration, frequency et al. Traditional electroporation is based on a kilovolt pulse generator which is not only bulky and costly, but also dangerous. Most importantly, the poration efficiency rarely exceeds 50%. So, the fundamental dynamics for poration and healing of individual cells are buried in an ensemble of both porated and unporated cells. By contrast, single-cell electroporation based on a microfluidic setup allows poration and healing dynamics of individual cells to be directly observed. In this study, multimodal characterization of single mammalian cells was carried out by optical and microwave techniques simultaneously during electroporation. Using a coplanar waveguide with a Jurkat cell trapped in the middle of its center conductor, continuous waves at 100 kHz of different amplitudes were applied for 20 s to porate the cell while microwave transmission coefficients at 9 GHz were measured every 0.4 s. The onset of electroporation was indicated by abrupt changes in both fluorescence intensity and transmission coefficient. Additionally, in measurements that lasted 300 s, the transmission coefficient was found to recover to the pre-poration level, while the fluorescence intensity remained different. Since the cells were confirmed viable through post-poration staining, the recovery of the transmission coefficient suggested reversible electroporation. These experimental results show that the transmission coefficient could serve as a label-free indicator of cell membrane permeability during and after electroporation. Furthermore, it can be used to expeditiously differentiate reversible electroporation from the irreversible one. This study should aid fundamental analysis of cell physiology, as well as molecular delivery in cell engineering and electrotherapy.