Phillip J. Hendrickson, Gene J. Yu, Dong Song, and Theodore W. Berger, University of Southern California, USA
Developing large-scale, quantitatively based models of neural systems has become a realizable goal in recent years, largely because of three major developments: (i) the accumulation of substantial knowledge of anatomical and physiological properties for many neural systems; (ii) the development of parallelizable software systems for representing these anatomical and physiological characteristics; and (iii) the continued growth of high performance computing systems capable of sustaining the numerical burden of simulating such large-scale models.
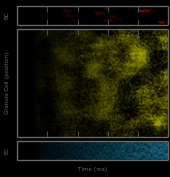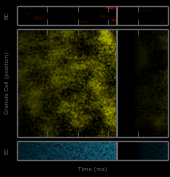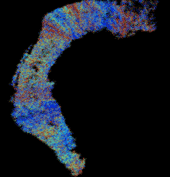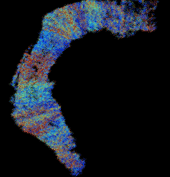
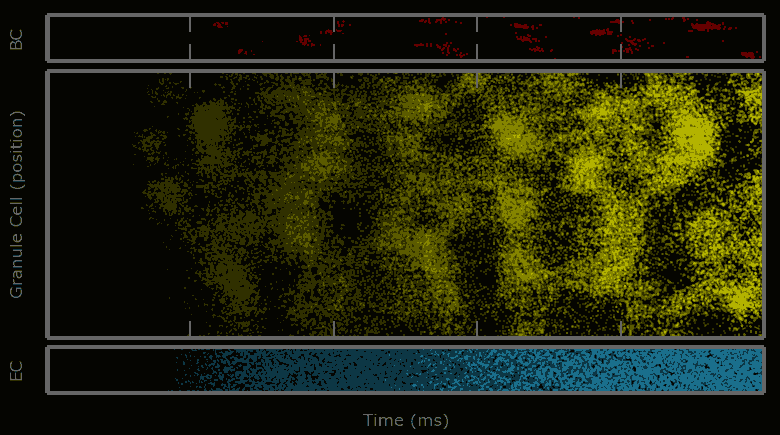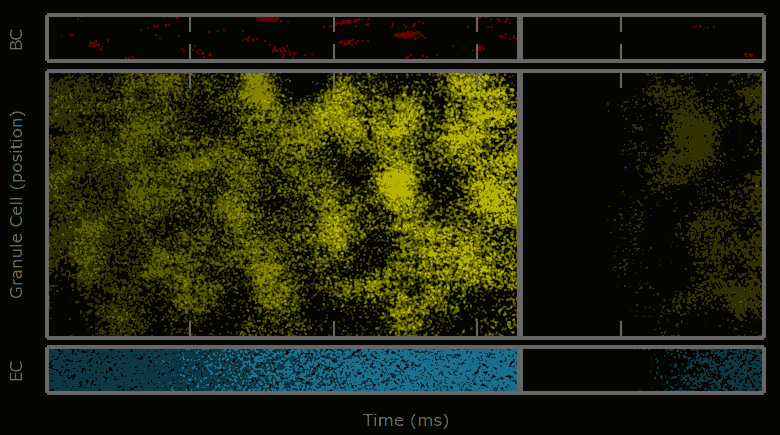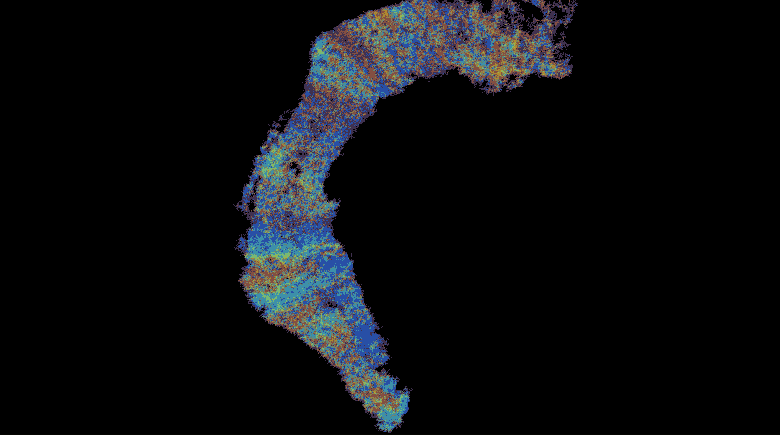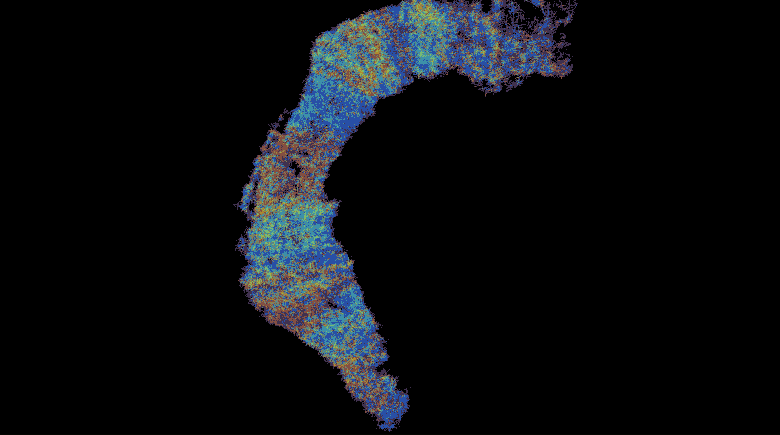
This work describes a million-plus granule cell compartmental model of the rat hippocampal dentate gyrus, including excitatory, perforant path input from the entorhinal cortex, and feedforward and feedback inhibitory input from dentate interneurons. The model includes experimentally determined morphological and biophysical properties of granule cells, each cell being composed of approximately 200 compartments. Modeling excitatory input from the entorhinal cortex was guided by axonal transport studies documenting the topographical organization of projections from subregions of the entorhinal cortices.
Results showed that when entorhinal cortical neurons maintained Poisson random firing, dentate granule cells throughout the network expressed a robust pattern of structured spiking best described as spatio-temporal “clustering.” To identify the network property or properties responsible for generating such “clusters”, we progressively eliminated from the model key mechanisms such as inhibition, membrane properties underlying rhythmic burst firing, and/or topographical organization of entorhinal afferents. Findings conclusively identified topographical organization of inputs as the key element responsible for generating a spatio-temporal distribution of clustered firing.
These results uncover a functional organization of perforant path afferents to the dentate gyrus not previously recognized: topography-dependent clusters of granule cell activity as “functional units” that organize the processing of entorhinal signals. They also reveal for the first time how a global signal processing feature of a neural network can evolve from one of its underlying structural characteristics. Further information can be found at http://cne.usc.edu.
Keywords: cluster analysis, compartmental model, dentate gyrus, dynamics, entorhinal cortex, gamma, hippocampus, mathematical model, model, neuroanatomy, oscillations, rhythmicity, spatio-temporal pattern, spike, synchrony, topographical organization, topography