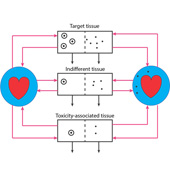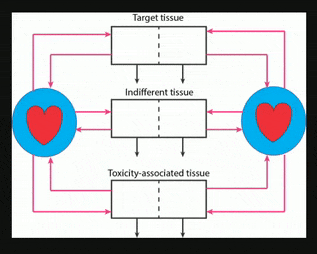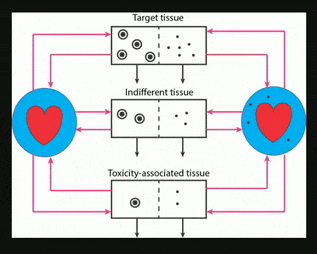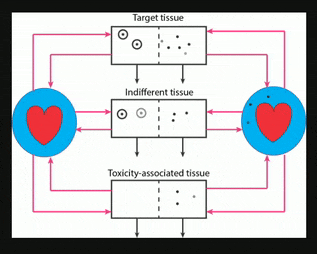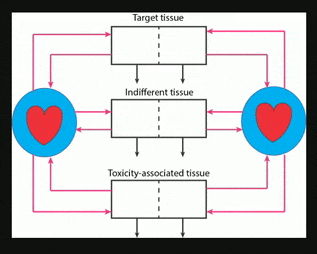
Nanocarriers, including solid nanoparticles, liposomes, dendrimers, and polymer micelles, are regarded as promising vehicles for targeted drug delivery, particularly for anticancer agents. Here, an in vivo kinetic framework is introduced to analyze and predict the quantitative advantage of using nanocarriers to deliver drugs, especially anticancer agents, compared to administering the same drugs in their free form. This framework recognizes three levels of kinetics. First is the particokinetics associated with deposition of nanocarriers into tissues associated with drug effect and toxicity, their residence time distributions inside those tissues, and elimination of the nanocarriers. Second is the release pattern in time of free drug from the nanocarriers. Third is the pharmacokinetics of free drug, as it relates to deposition and elimination processes in the target and toxicity associated tissues, and total body clearance. A drug targeting index (DTI) is defined, quantitating the benefit of using nanocarriers by considering the effects of preferential deposition of nanoparticles into target tissues and relative avoidance of tissues associated with drug toxicity, compared to drug that is administered in its free form. General methods are derived for calculating DTI when appropriate particokinetic, pharmacokinetic, and drug release rate information is available, and it is shown that relatively simple algebraic forms result when some common assumptions are made. This approach may find use in developing and selecting nanocarrier formulations, either for populations or for individuals.