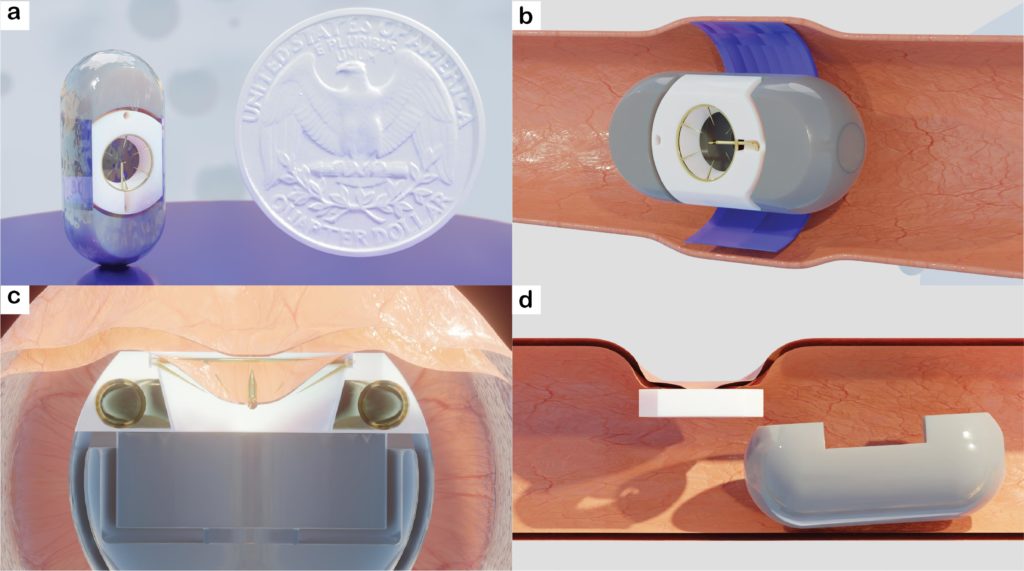
Biological drugs (biologics) are derived from living organisms such as humans, animals, or microorganisms. Compared with conventional synthetic chemical drugs, biologics are relatively large and complex molecules, made up of proteins, carbohydrates, nucleic acids, or a complex composite of these substances. Different research groups have introduced various ingestible devices or capsule robots for the intraluminal delivery of drugs. However, most of the recent work focuses on delivery methods where the drug is physically or chemically infused with the delivery mechanism. Conversely, an approach to deliver unmodified biological drugs can eliminate the cost and complications associated with developing new drug formulations. In the current work, we introduce a swallowable capsule for intestinal drug delivery (SCIDD) to deliver physically and chemically unaltered adalimumab, a biological drug, into the submucosa layer of small intestine. The design, optimization, and validation of the SCIDD’s primary subsystems were performed both ex-vivo and in-vivo. The assembled capsule was further tested in vivo to validate the actuation sequence and showed a 70% (n=17) success rate in an animal model. Additionally, a drug delivery study indicated systemic uptake of adalimumab via SCIDD compared with luminal delivery in the small intestine. The pilot study presented here establishes that the novel platform could be used to orally deliver systemic biologics.
