Type B aortic dissection (TBAD) is a serious clinical emergency characterized by a split in the layers of the aortic wall which allows blood to enter a second channel known as false lumen. This disease impairs blood supply to body organs and may lead to further complications such as aortic aneurysm, rupture or malperfusion syndromes. As of today, thoracic endovascular repair (TEVAR) is one of the most promising treatments for TBAD.
Most computational models of TBAD involve varying degrees of simplifications, due to the lack of patient-specific flow and pressure measurements, and/or of a proper workflow taking full advantage of available in vivo data. The aim of this study was to develop a truly patient-specific computational methodology for the evaluation of blood flow in TBAD, before and after TEVAR.
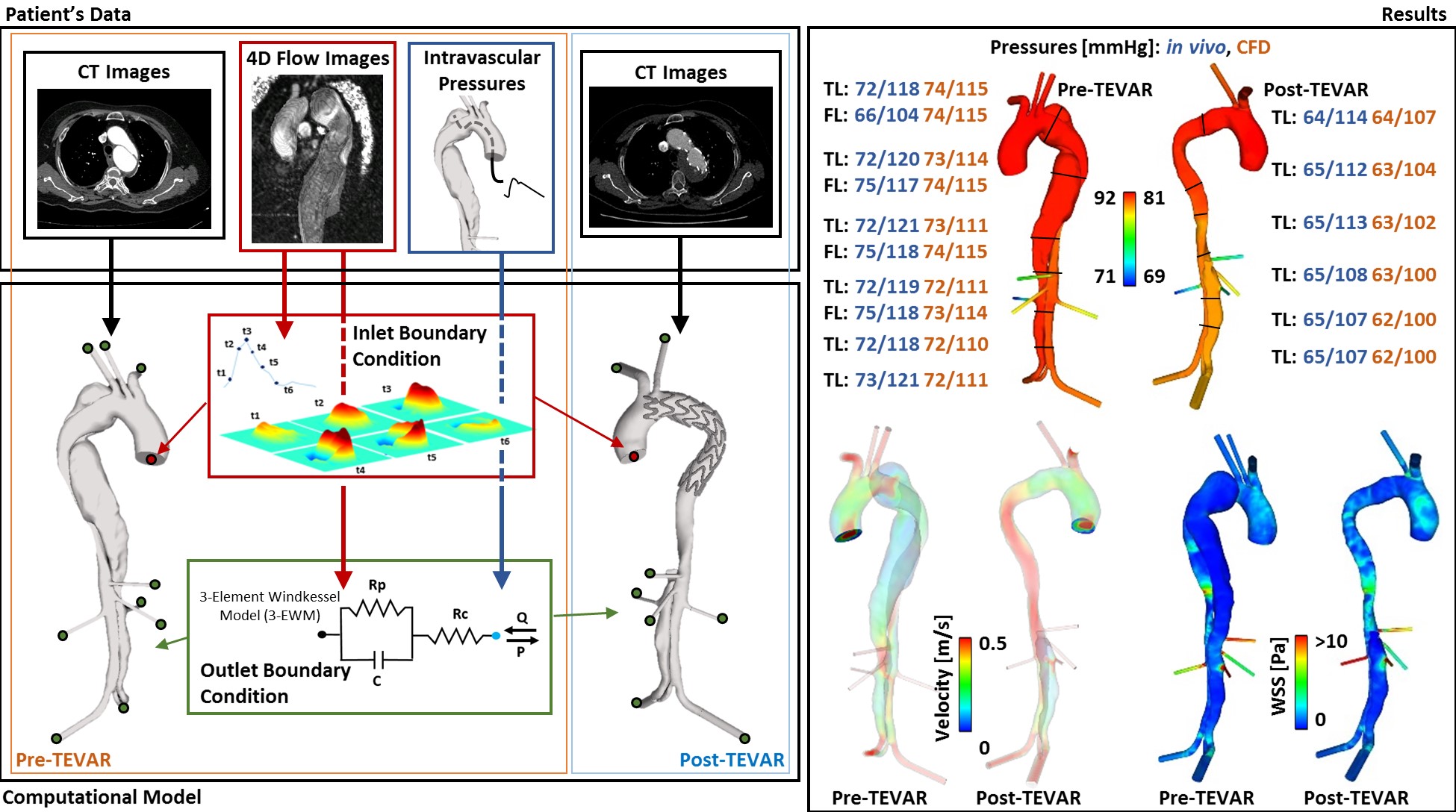
The TBAD model geometry was reconstructed from CT images, including multiple visceral branches in the abdomen. 4D flow MR images acquired from the same patient were analysed to extract time-varying velocity profiles at the model inlet. 4D flow and the corresponding pressure data were combined to tune the Windkessel parameters for the model outlets. In the paper, the workflow followed to obtain and prescribe such boundary conditions is described in detail, together with the derived parameter values which can be applied to other patient-specific simulations after appropriate tuning.
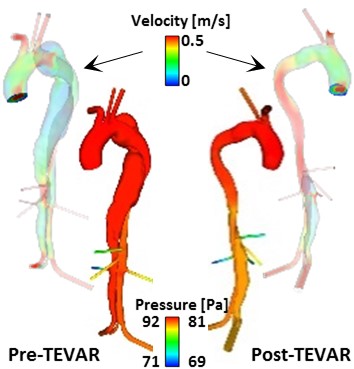
Computational results for the pre-TEVAR model were validated against the corresponding in vivo measurements, showing a good overall agreement. The extracted boundary conditions were then used to evaluate aortic hemodynamics in the same patient after TEVAR, and the model correctly predicted the reduction in true lumen pressure post-TEVAR. This study demonstrates that post-TEVAR hemodynamics can be evaluated by using boundary conditions derived from pre-TEVAR flow and pressure data, without the need for post-TEVAR flow measurements which are usually difficult to obtain.