Imagine a transparent chip the size of a flash drive. In it, your own cells—derived from a skin sample—are grown in delicate channels to mimic your heart, lung, or even brain tissue, creating a testing ground for personalized medical treatments.
Though this is a hopeful vision of the future, the reality may not be too far off. Organs-on-chips, also known as OOCs, may evoke images of Frankenstein-like flesh stitched together on a circuit board but, in reality, this technology involves precise and often elegant tools (with one so well designed it was acquired by the Museum of Modern Art). Researchers have worked on such microphysiological systems for many years, but the technology has recently captured more widespread interest. The U.S. Federal Food and Drug Administration (FDA), biotech, pharmaceutical companies, and academic laboratories are investing in OOCs as part of a suite of emerging laboratory tools—such as organoids—that offer sophisticated ways to model human biology.
Many of these microfluidic chips consist of artificial polymers built with networks of channels and membranes, on which cells are grown to mimic functions of an organ. The chips tend to be optically transparent (so researchers can observe the molecular and cellular forces at work); flexible (so they can recreate mechanical forces associated with blood and air flow, and to mimic organ motions, like breathing or peristalsis); and compatible with computational instruments (to precisely flow in chemicals like therapeutics or toxins). With these attributes, OOCs have the potential to accelerate clinical trials, minimize the need for animal testing, and even usher in a new era of personalized medicine.
Facilitating drug discovery
By imitating human biological functions, organ chips may provide a critical missing link to speed up drug discovery—both in terms of designing new drugs, as well as testing whether existing drugs might be useful for applications beyond what they were initially approved for.
“We can mimic human responses in ways never before to discover new mechanistic insights and develop drugs more quickly,” says organ chip pioneer and Wyss Institute Founding Director Donald Ingber, M.D., Ph.D. “The sheer level of the quality of results you get from these chips can be much more relevant compared to existing in vitro models and animal models.”
One of Ingber’s chips is designed to imitate the lung. On it, lung airway cells grow in one channel. On the other side of a porous membrane, cells making blood vessels reside in a second channel full of flowing liquid with blood-like consistency, akin to components of the lung environment. Cells differentiate similar to how they would in a human airway and even develop relevant traits, like producing and clearing mucus, according to the institute.
“We’re finding these chips mimic clinical results,” says Ingber. “We’ve repurposed existing drugs for COVID-19 using chips, and we’re developing new drugs by applying sophisticated computational approaches including rational drug design and machine learning.”
When scientists around the world began mining for existing drugs that might treat COVID-19 in May 2021, for example, Ingber’s team used their OOC to help identify the antimalarial drug amodiaquine as an inhibitor of infection with SARS-CoV-2, as reported in Nature Biomedical Engineering. In the study, they investigated about 30 existing drugs that had been reported to show some activity against related viruses in the past and found eight of these showed promise as inhibitors of SARS-CoV-2 entry into cells in conventional tissue cultures. Because the chip more precisely reflects organ level structures and functions of a human lung, they were able to home in on amodiaquine. They demonstrated that it blocked viral entry, paving the way for it to head into clinical trials.
New insights into diseases
After founding one of the first OOC companies, called Emulate, nearly a decade ago, Ingber and others have continued to push the field forward. Emulate has partnered with companies such as Johnson & Johnson and Merck to develop chips based on major organs of the body. Additionally, Ingber’s laboratory is using this technology to mimic lymph nodes to test human vaccine reactions; model rare genetic disorders directly from patients; and glean insights into the microbiome (Figure 1). “We have at least 15 models here,” says Ingber. “We haven’t found an organ yet that we couldn’t model.”
As one example, the Wyss Institute recently announced a chip that recapitulates—more accurately than other in vitro systems—aspects of cystic fibrosis, a disease that causes mucus in airways to thicken, leading to infections, and ultimately respiratory failure. This new organ chip provides a preclinical human model to help facilitate much-needed treatments (Figure 2).
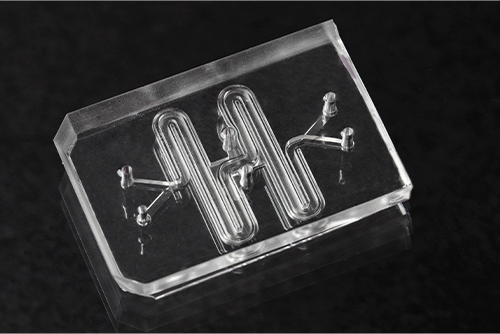
Other groups across the globe are likewise developing OOCs to better understand medication and diseases, from modeling the brain-blood barrier to studying cancer metastases to treating life-threatening disorders. Clive N. Svendsen, Ph.D., professor and executive director of the Cedars-Sinai Board of Governors Regenerative Medicine Institute (RMI), for example, partnered with Emulate to create functioning tissues of spinal cord, intestines, and kidneys for research and drug development. Svendsen’s laboratory at Cedars-Sinai aims to develop these to further model Parkinson’s disease, amyotrophic lateral sclerosis (ALS), ovarian cancer, and inflammatory bowel disease.
Another effort, this one at the Columbia University, has made progress in exploring cancer and tumor drugs. Gordana Vunjak-Novakovic, Ph.D., is a bioengineer and university professor who has made significant advances in OOCs. She published a recent paper demonstrating that engineered human tumor and cardiac tissues on her OOC integrated platform were able to accurately predict a new antitumor drug’s effect.
“With the advances in stem cell biology and tissue engineering, these models can be patient-specific and are increasingly capable of recapitulating organ level functions and systemic cell and tissue responses,” says Vunjak-Novakovic. “An example of such an emerging application is the incredibly important and complex conditions associated with cancer metastasis.”
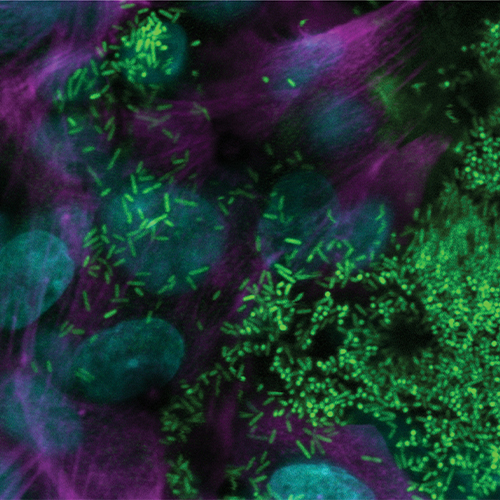
In addition to testing drugs and evaluating disease, many are hoping OOCs improve the process of clinical trials. In fact, the National Institutes of Health (NIH) is betting on it.
Speeding up clinical trials
According to the NIH, a disheartening 85% of candidate drugs in late-stage clinical trials fail because of safety or ineffectiveness, despite promising initial results. In 2020, NIH’s National Center for Advancing Translational Sciences (NCATS) announced their Clinical Trials on a Chip Program, through which they awarded ten grants totaling US$35 million to efforts focused on using tissue chips to improve the efficiency of late-stage clinical trials of investigative drugs.
“This new program has the opportunity to improve how we conduct clinical studies and their use for precision medicine,” says Danilo A. Tagle, Ph.D., director of the Office of Special Initiatives of NCATS. “We have a timeline of about five years to see how best OOC can be effectively used in these studies.”
Awardees include Deok-Ho Kim, Ph.D., and David Kass, Ph.D., at the Johns Hopkins University, who are focused on modeling dystrophin-deficient muscular dystrophy; Y. Shrike Zhang, Ph.D., and Marie Denise Gerhard-Herman, M.D., at Brigham and Women’s Hospital, who are engineering a tissue model of the genetic premature aging disorder Hutchinson-Gilford progeria syndrome; Svendsen’s group at Cedar Sinai, who is homing in on ALS; and others.
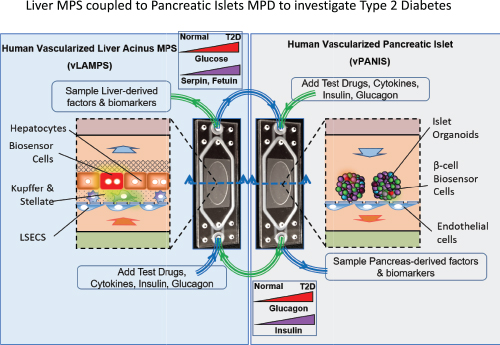
One awardee, D. Lansing Taylor, Ph.D., director of the University of Pittsburgh’s Drug Discovery Institute, developed human biomimetic chips that recapitulate liver functions with a vascular channel using one pump and a hepatic channel with another pump. His group is modeling nonalcoholic fatty liver disease and type 2 diabetes both in single liver chips and connected liver and pancreatic islets chips, measuring conditions like insulin resistance, inflammation, and fibrosis (Figure 3).
“One of the biggest potential impacts of this technology is to create a preclinical trial platform using patient-derived cells,” says Taylor.
Organ chips wouldn’t just make clinical trials optimal by identifying patient cohorts and by being less expensive for researchers and companies; they could also help prevent suboptimal treatments for patients, he points out. Scientists could discern if a drug is more likely to work with a patient’s disease state and genetics after customized organ chip testing and wouldn’t have to put them through a whole clinical trial that might not yield results. “If we could test drugs before they go into clinical trials, we can help define patient cohorts so there’s a higher probability the drug will work in the clinic,” he says.
Additionally, OOCs could supplement—and maybe one day replace—animal testing. Researchers are already developing organ chips of rat and dog liver cells in the hopes of replacing required preclinical animal tests for new drugs.
“Animal models are not optimal for toxicity or disease models,” says Taylor. “They have serious scientific limitations because they are not the same as human physiology, plus there is great pressure from society to minimize the use of animals for testing.” His group hopes to have their preclinical trial platform built and ready for use within five years.
Organ chip boom
In addition to drug development and clinical tools, OOCs have far-reaching uses in other areas, such as understanding fundamental processes like aging, or gleaning insights into how to deal with environmental threats.

As one example, through partnerships with NASA and the International Space Station U.S. National Laboratory, OOCs have been sent to space to study the accelerated decline seen in muscles, bones, and the cardiovascular and immune system in zero or microgravity. By using OOCs to observe the accelerated aging process, scientists hope to better understand detrimental effects often associated with aging—and how to stop it.
Organ chips could also help accelerate responses to environmental hazards. The Wake Forest Institute for Regenerative Medicine (WFIRM) established a lung-on-a-chip platform as a clinically relevant model of chlorine gas exposure to humans (Figures 4 and 5). Exposure to chlorine gas, whether through chemical attacks or accidents in the home or workplace, can quickly become fatal.
“This chlorine-gas injury to the lung-on-a-chip mimics the type of injury that happens if humans were to breathe in chlorine gas, including acid formation on the lung surface, death of the lung cells which worsens over time, and a breakdown of the barrier between the air and blood,” says project lead Sean Murphy, Ph.D., associate professor of regenerative medicine at WFIRM. “The next steps of this work are to better understand the injury pathways that are occurring after exposure and the identification and testing of potential drugs that could prevent this type of injury.”
Some groups are looking at increasing the complexity of OOCs to bolster its abilities. Draper, Cambridge, MA, USA, has developed what it calls a “human-organ-system” named PREDICT96, that has 96 independent samples and 192 micro-pumps, which is far more components than most OOCs.
“OOCs are a game changer for developing new therapies,” says Timothy Petrie, Ph.D., head of strategy and business development, pharmaceutical research and development technologies at Draper. “Currently, however, most OOC systems are configured as laboratory research tools and typically only house single digit human organ tissue replicates at a time. Draper designed its highly scalable HOS platform so that researchers can test hundreds of biologics simultaneously on individual and independent miniature tissue organ models.”
With this increased complexity, Draper aims to offer a realistic human organ environment to discover, test, and improve drugs, especially for the liver, kidney, and gut. In September 2021, Draper was awarded over US$700,000 by the U.S. Department of Health and Human Services’ ImmuneChip+ program to adapt this technology into a version called PREDICT96-AIR. PREDICT96-AIR will focus on how coronavirus and other respiratory diseases affect primary airway epithelial cells and supporting immune tissue—and potential therapies to treat these diseases.
Remaining barriers
Despite its myriad uses, organ-on-chip technology is faced with several obstacles slowing its adoption. Cultivating cells and tissues to reach adult-like, molecular, structural, and functional tissue properties, and maintaining these properties for months, is one critical area for improvement, according to Vunjak-Novakovic. Availability of standardized, configurable OOCs at affordable cost and their continued validation against clinical data are also essential for widespread adoption.
Tagle adds that additional hurdles include gaining renewable and reliable cell sources from individuals, as well as making technical improvements for biocompatible materials. To address this, NCATS is partnering with several agencies to continue to improve OOC technology and to automate and miniaturize the instrumentation for OOC experiments. “I think the future is bright for OOCs for use as a drug development tool by pharma, as a tool for regulatory decision making by the FDA, and as validated model systems for studying and seeking cures for numerous human diseases,” says Tagle.
Vunjak-Novakovic adds that she believes these systems will ultimately lead to personalized medicine becoming broadly available, but it will take some time. “These ambitious goals can only be accomplished through the effective joint efforts of many disciplines,” she says. “As we live in an era of collaboration, I am optimistic that we will see major progress in the coming years.”



