“The main facts in human life are five: birth, food, sleep, love, and death.”
—E.M. Forster
Despite the fact that we spend nearly one third of our lives asleep, surprisingly little was known about sleep until the 20th century. Now, sleep medicine is firmly established as a significant branch of medical practice, taking its roots strongly from the work of Nathaniel Kleitman and colleagues at the University of Chicago in the 1950s. They were the first to show the existence of rapid eye movement (REM) sleep—commonly associated with dreaming—and began the process of opening our eyes to the complex physiological processes that occur during sleep.
The field progressed in the 1960s, with an increasing standardization of physiological signal recording that led to the current standard for sleep measurement—the polysomnogram (PSG). This is a test in which a technician places multiple electrodes on a patient (including, at a minimum, electroencephalogram, electrocardiogram, airflow, respiratory effort, and oximetry sensors) during a night’s sleep at a sleep laboratory. The data obtained from such a test is comprehensive and can provide great insight into many different sleep disorders. However, the PSG is also limited, as it captures only a single night of data in an artificial environment and is unlikely to represent a “normal” night for the subject being tested. Hence, there has been continued interest in developing sleep measurement technologies that can provide useful information about sleep, over multiple nights, and with minimal interference to the subject.
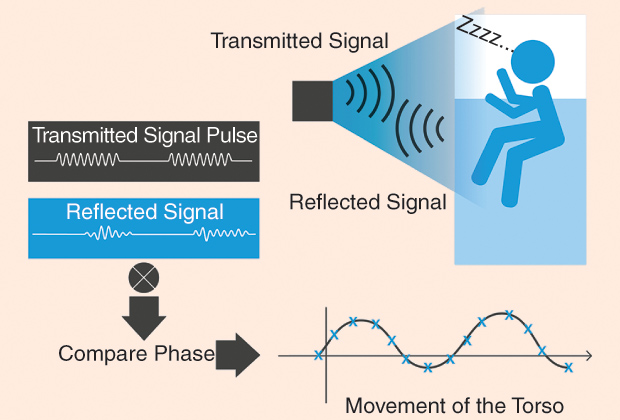
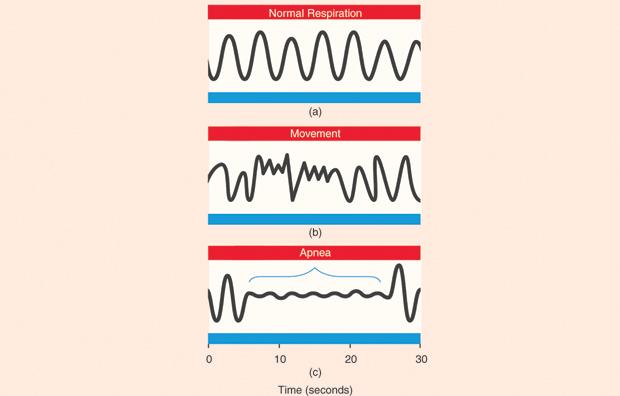
One technology that shows a lot of promise in this area is radio-frequency (RF) biomotion sensing of sleep. The basic concept of biomotion sensing is quite straightforward and builds on the long history of RF sensing used in radar technology. Figure 1 shows the basic concept of a biomotion sensor to measure sleep. A short pulse of radio waves is transmitted from the transmitter. This is reflected off the subject, and the echo signal is received back in the receiver. By comparing the phase of the transmitted and reflected signals, any movement of the target (the person’s torso) can be detected. Accuracy and noise suppression can be improved by transmitting multiple pulses and averaging together the phase differences. The phase differences can then be sampled at a suitable rate (e.g., 16 Hz) to detect both respiration and bodily movements. In terms of analyzing the sleep of a person, there are two main frequency bands of interest to detect: 1) respiratory movements, which are typically between 0.15 and 0.4 Hz, and 2) more general body movements, such as turning over (which are higher in frequency). Figure 2 gives an example of three different types of signals that can be obtained by a noncontact biomotion sensor. Figure 2(a) shows regular breathing, normal during deep sleep. Breathing interrupted by a body movement (changing position) is shown in Figure 2(b). Figure 2(c) illustrates a clinical event called an obstructive apnea, where a person’s upper airway is obstructed, but the chest continues to make small movements as the person tries to breathe.
For the last several years, our research team has focused on producing a biomotion sensor, which is practical for use in home and lab-based sleep measurement (the system is called SleepMinder). There were several factors of practical concern to consider when designing an RF biomotion sensor. First, as the device is intended for widespread use in many countries, it is good to select an available Industrial Scientific and Medical frequency band—in our case, we developed systems that work at 5.8 and 10.525 GHz as these are license-free bands in many countries, provided the designer stays within frequency and power limitations. At the frequencies we have chosen, the maximum allowed emitted power is typically 10 mW, and in the systems we have developed, we typically use an equivalent isotropically radiated power (EIRP) of approximately 1 mW (0 dBm). This also means that the system’s emitted power falls orders of magnitude below international safety standards for nonionizing radiation (for comparison, a cell phone can emit a maximum EIRP of up to 2,000 mW). Fortunately, normal bedding and clothing is relatively transparent for radio waves in the gigahertz region, so the quality of the signal is unaffected by these factors. Note also that the penetration depth of a 5–10-GHz RF signal in the skin is about 0.4–1 cm, so the received signal primarily reflects movement of the skin surface (and not internal phenomena such as lung or heart movement).
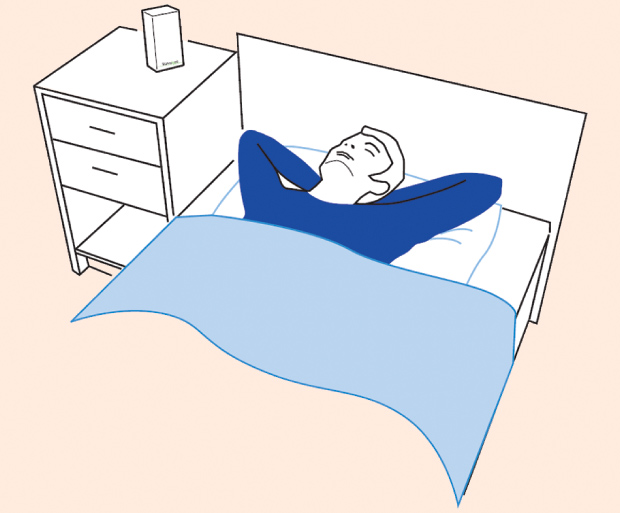
An important factor to consider is the orientation of the subject with respect to the sensor. Figure 3 shows an example of the sleep sensor on a bedside table. It is very typical for people to change position often during sleep, moving between supine, face-down, left- and right-side positions. Since the sensor is effectively picking up respiratory movements of the torso in the direction normal to the sensor orientation, the amplitude of the received signal will depend on the orientation. However, fortunately, even with the subject’s back facing the sensor, our experiments have shown that there is still sufficient movement to detect in the majority of cases.
Another critical influence on signal quality is the distance of the subject from the sensor; typically, the received power will fall off as  1/r^a, where
1/r^a, where  a is between two and four. In practice, we have found that adequate signals can be obtained up to 1.2 m from the subject, making the system ideal for placing on a bedside table. To ensure that the sensor primarily detects only signals from the nearest subjects, it was designed with a horn antenna that provides a restricted beamwidth of ±30°. Finally, since the majority of adults have a bed partner, the SleepMinder includes a feature called range-gating, in which only reflections from objects to up to a certain distance are obtained (this is achieved by only sampling the reflected signal during a certain time window).
a is between two and four. In practice, we have found that adequate signals can be obtained up to 1.2 m from the subject, making the system ideal for placing on a bedside table. To ensure that the sensor primarily detects only signals from the nearest subjects, it was designed with a horn antenna that provides a restricted beamwidth of ±30°. Finally, since the majority of adults have a bed partner, the SleepMinder includes a feature called range-gating, in which only reflections from objects to up to a certain distance are obtained (this is achieved by only sampling the reflected signal during a certain time window).
Of course, obtaining a good movement signal is only half the battle. We have developed algorithms that can map the movement signal into useful information about sleep and respiration. The sleep/wake distinction can be primarily made on the basis of movement; if a person is continuously moving, they are unlikely to be asleep! The field of actigraphy (which uses accelerometers mounted in wristwatches to observe movement during sleep) has already developed algorithms to map movement patterns to likely sleep state, so we have adapted concepts from that field. In addition, the biomotion sensor provides information about respiration. It has been already shown that the deepest stages of sleep are associated with very regular respiration patterns, whereas REM sleep is characterized by breathing that is variable in both amplitude and rate. By collecting together both the movement and the respiration patterns, we have developed a machine learning algorithm that maps the biomotion sensor to sleep state.
In studies where the sensor and algorithm are compared with the gold-standard PSG measurements, the noncontact system agrees with the sleep/wake classification of the PSG more than 85% of the time. This is comparable with the best actigraphy systems. Moreover, since the system can measure respiratory effort, it can be used to identify apnea and hypopnea events with a good degree of accuracy. In a study of 74 subjects suspected of having sleep apnea, the noncontact sensor system was 90% sensitive and 92% specific in recognizing patients with and without sleep apnea, using the standard cutoff of an Apnea Hypopnea Index greater than 15 to define sleep apnea.
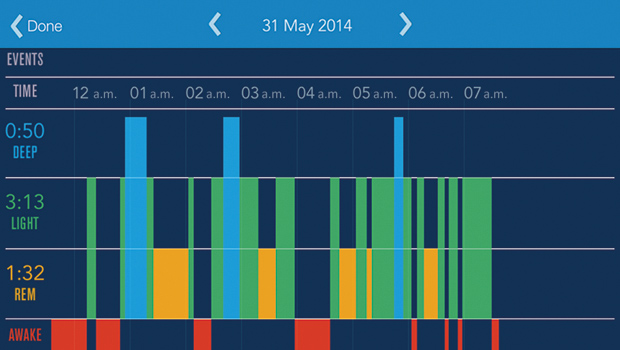
So what are the potential uses of a noncontact sleep assessment system? First, the last few years have seen the first generation of wellness self-assessment devices (the so-called Quantified Self movement) to reach broad consumer appeal. For example, the Fitbit wristband has proved popular with consumers for assessing their daily activity levels; likewise, the BodyMedia FIT armband has found popularity as an aid to weight reduction, by providing quantitative information on calorie expenditure. Many common sleep issues are nonmedical in nature and are contributed to by poor knowledge about sleep and sleep hygiene. (Sleep hygiene refers to good habits around sleep, such as aiming to go to bed and get up at the same time, avoiding stimulants late in the day, allowing a wind-down period before sleep, etc.) The noncontact monitor provides a convenient way for consumers to track their own sleep without any need for wearing wristbands, chest bands, etc. Combined with other information about their daily habits and environment, accurate sleep measurements allow people to take better control of their own sleep patterns. Figure 4 shows an example of a hypnogram obtained from the author, which uses a combination of movement and respiration information to determine wake, light sleep, deep sleep, and REM sleep.
On the clinical side, the technology can be used to identify or track the incidence of sleep-disordered breathing. This may be helpful in screening subjects for sleep apnea or for tracking people’s response to conservative therapies for mild sleep apnea, such as weight loss or mandibular repositioning devices. The device may also be useful in chronic medical conditions such as congestive heart failure and chronic obstructive pulmonary disease, where the presence and variability of sleep apnea from night to night may be a useful clue to the underlying disease itself. Since the device can also track respiration rate, it can be used in a chronic disease management system, where the respiration rate provides a clue to the underlying disease process (such as in chronic obstructive pulmonary disease).
To summarize, our goal has been to simplify the process of sleep and respiration measurement, allowing continuous monitoring over multiple nights—permitting individuals to understand their own sleep patterns or enabling medical professionals to provide improved care and guidance to individuals suffering from a number of sleep and respiratory disorders. The ongoing challenge is to further improve the accuracy and sensitivity of the technology and, ideally, to add in further information without compromising the convenience and noninvasiveness of the overall system from a user’s point of view.
References
- P. E. Peppard, T. Young, J. H. Barnet, M. Palta, E. W. Hagen, and K. M. Hla, “Increased prevalence of sleep-disordered breathing in adults,” Am. J. Epidemiol., vol. 177, no. 9, pp. 1006–1014, May 2013.
- P. deChazal, N. Fox, E. O’Hare, C. Heneghan, A. Zaffaroni, P. Boyle, and W. T. McNicholas, “Sleep/wake measurement using a non-contact biomotion sensor,” J. Sleep Res., vol. 20, no. 2, pp. 356–366, 2012.
- E. O’Hare, D. Flanagan, T. Penzel, C. Garcia, D. Frohberg, and C. Heneghan, “A comparison of radio-frequency biomotion sensors and actigraphy versus polysomnography for the assessment of sleep in normal subjects,” Sleep Breathing, to be published.
- A. Zaffaroni, B. Kent, E. O’Hare, C. Heneghan, P. Boyle, G. O’Connell, and W. T. McNicholas, “Assessment of sleep-disordered breathing using a non-contact bio-motion sensor,” J. Sleep Res., vol. 22, no. 2, pp. 231–236, 2013.



