Elite-level athletes and professional sports teams are continually searching for opportunities to improve athletic performance and gain a competitive advantage on the field. Advances in technology have provided new avenues to maximize player health and safety. Over the last decade, time–motion analysis systems, such as video recording and computer digitization, have been used to measure human locomotion and improve sports performance. While these techniques were state of the art at the time, their usefulness is inhibited by the questionable validity of the acquired data, the labor-intensive nature of collecting data with manual hand-notation techniques, and their inability to track athlete position, movement, displacement, and velocity.
The advent of the global positioning system (GPS) and accelerometer technology has changed the landscape to allow more efficient and accurate measurement of athletic movements. Currently, two types of GPS devices exist, differential and nondifferential, with the former being used to obtain speed, position, and distance measurements and the latter preferred due to its low cost, form factor, and simplified data analysis [1]. GPS now plays an instrumental role in sports performance analysis by allowing coaches, physicians, and trainers to better understand the physical demands made on an athlete in real time. GPS silicon chips combined with triaxial accelerometers help objectively record physical activities conducted at different times of the day and for specific position groups on a team [2], [3].
The most common types of accelerometers are piezoresistive, piezoelectric, and differential capacitive. Differential capacitors are the most utilized in sports due to their ability to classify posture, movement, energy expenditure, and balance control [3]. The combination of GPS, accelerometer, and heartrate technology into one sensor has provided team physicians and trainers with a multimodal platform to monitor the energy cost and specificity of movement patterns over the course of a game or practice [2]. Most commercially available integrated technology (IT) devices using this platform now contain inertial measurement units, which are housed in a miniature case and worn in a small purpose-built pocket in the back of a jersey or strapped onto an athlete [2], [3]. These devices, which we refer to here as wearable devices, allow for real-time movement and load measurements of athletes during practice and alleviate the need for laborious video recording, as was done previously [3].
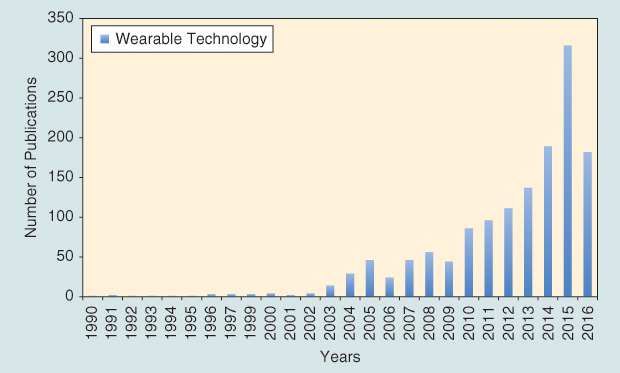
This area has seen an exponential increase in published literature over the last decade (Figure 1). Here, we highlight a few applications for the growing wearable device field that detect various biomarkers (impact forces, stress, and strain) and biovitals (sweat and temperature) for sports medicine. No longer are wearable devices a novelty. Progress in wearable sensors, analytics, and reliability has positioned the field for translation into the clinical setting.
IT
Commercially available devices, such as the Catapult Optime- Eye with its custom-developed Minimaxx analytics platform, have been used to describe the physical movement demands in rugby, the Australian Football League, the National Football League (NFL), and the National Hockey League, among others [2]. Catapult devices are capable of measuring over 262 parameters and collect roughly 1,000 data points per second, with the key data points being player load, player load per yard traveled, and player load per time, where time refers to the duration of the session. The measure of exertion detected is not dependent on the distance a player travels, as sports like rugby or football have workloads over and beyond running. The two primary equations utilized by Catapult devices to calculate player load are shown in (1) and (2), where PL is the player load, AccPL is the accumulated player load, fwd is forward acceleration, side is sideways acceleration, and up is upward acceleration.
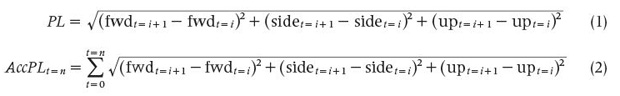
Another device that has been used in the National Basketball Association and during the 2016 Summer Olympics in Rio de Janeiro, Brazil, is the Whoop wristband, which measures heart rate, body temperature, and body movements. The data generated from IT-based wearable devices provide baseline information that may aid in the development of performance programs or injury prevention strategies based on player position [2], [3]. A recent study from September 2015 to January 2016 conducted by Whoop, which monitored 119 athletes among eight National Collegiate Athletic Association Division I teams across different sports, showed a 60% reduction in injuries for the athletes who used the device and acted upon the acquired data [4]. The study showed an improvement in sleep time and quality, a decrease in resting heart rate and heart-rate variability, and an improvement in key biomarkers and stress [4].
Research using IT in team sports up to this point has been primarily conducted in outdoor settings because GPS units (so far) are unable to track movement patterns indoors. Because of this issue, the use of IT has been limited to field-based team sports. Previous studies have applied IT to determine the speed, position, and distance of athletes during practices and games. Current studies have expanded on this work to measure and interpret physiological data, such as heart rate and magnitude and frequency of contact. To further minimize and prevent injuries in sports, there is a need to develop a multimodal wearable device capable of accurately detecting both biomarkers and biovitals.
Epidermal-Based Wearable Devices
Soft-tissue injuries remain the most common type of injury among athletes, with the most severe types producing chronic pain and dysfunction and leading to recurrence, all of which ultimately result in missed time from sport. These injuries are often caused by poor conditioning, overtraining, or dehydration [5]–[7]. The next generation of wearable devices could be designed to decrease this major injury type via the measurement of appropriate biovitals from, for example, sweat.
Sweat analysis—currently used for disease diagnosis, drug abuse detection, and athletic performance optimization [5], [6]—is now only possible through a probe that depends on direct chemical concentration with the analyte (dissolved compounds) [7]. Creating wearable chemical sensors that can identify and quantify biomarkers from sweat—such as electrolytes (sodium, chloride, potassium, and calcium), metabolites (lactate, creatinine, glucose, and uric acid), small molecules (amino acids, DHEA, and cortisol), and proteins (interleukins, tumor necrosis factors, and neuropeptides)—in a nonintrusive manner and relay corresponding vital signs and signals via Bluetooth to an external receiver appears to be extremely promising for sports, as detailed in Figure 2 [5], [6]. By measuring sweat, researchers and sports physicians can gain a more complete understanding of how physical activity and exertion relate to fatigue and injuries. Specifically, measurement of lactic acid, which is a byproduct of burning glucose without oxygen, provides a thorough indicator of an athlete’s ability to cope during rigorous exercise [6], [7].
![Figure 2: A schematic detailing an epidermal wearable sensor for analyte detection from sweat. Adapted and modified from [5]. Na+: detection of sodium.](https://www.embs.org/wp-content/uploads/2017/01/seshadri02-2627240.jpg)
A key challenge to this technology remains: How does one efficiently, accurately, and noninvasively measure the aforementioned analytes from the body? This challenge can be overcome via the measurement of eccrine sweat, which offers significant advantages and disadvantages, as highlighted in Table 1 [6]. Eccrine sweat glands are located in many parts of the body at high densities (>1,000 glands/cm^2) and thus provide opportune sampling spots [5]. On this front, the use of flexible electronics to create a sensor capable of adhering to the surface of the skin to measure and quantify analytes from sweat is of interest to and highly desired by the sports community.
Besides the issue of efficiency, these sensors must fulfill several other requirements: high stretchability, flexibility, and durability; low power consumption; biocompatibility; and lightness in weight. These requirements become imperative for epidermal electronic devices, where mechanical compliance and integrity with human skin are crucial for sensor functionality (i.e., the sensor material strain needs to exceed 100%) [8].
[accordion title=”Table 1. The Advantages and Disadvantages of Utilizing Eccrine Sweat as a Source for Analyte Detection (6).”]
| Key Advantages | Key Disadvantages |
| High density enables large sampling locations | Skin surface represents a large area for contamination |
| Constant access | Old sweat can mix with new sweat, thereby affecting data accuracy |
| Sampled without external contamination | Low sample production rates (nL/min/mm²) |
| Ease of sampling without analyte degradation | Large analytes, such as proteins, are filtered or diluted |
| Sampled at low concentrations | Device fabrication economics |
| Ease of placement and comfort (nonintrusive) | Impact forces on body could alter sensor–analyte interaction, thereby damaging the device |
[/accordion]
Nanomaterials are drawing increased attention as functional sensing elements due to their excellent electrical, mechanical, optical, and chemical properties. Polymers, such as polydimethylsiloxane, silicon, and thermoplastic elastomers, are used as flexible support materials because of their flexibility, stretchability, and durability [8]. Additionally, graphene, carbon nanotubes, nanowires, and hybrid micro-/nanostructures have been utilized to fabricate stretchable sensors [8]. This promising research and development can be applied toward concussion detection and management.
Concussion-Based Wearable Sensors
Approximately 1.6–3.8 million people suffer sports- or recreation-related brain injury in the United States annually [9]. Concussions remain a major issue in contact-laden sports, such as rugby and American football [10]. Recently, the NFL has initiated a procedure wherein a player suspected of experiencing a concussion during a game or practice is placed on a coordinated concussion protocol to ensure that he or she returns to the field in a safe and healthy manner after recovery.
Currently, wearable devices in the research and development stage can detect and monitor the impact force exerted on the head during contact activities [9]. The development of a wearable device to measure impact forces on the head is of great interest to minimize concussions and ultimately decrease the incidence of chronic traumatic encephalopathy—progressive degeneration of the brain due to repeated trauma— in athletes. Two devices that have shown promise in detecting and measuring impact forces on the head are the Linx IAS and Q-Collar [9].
Linx IAS is a wearable device that measures and records the force of hits taken to the head and sends real-time data to the appropriate personnel. It is a small, lightweight rectangular device placed inside a head- or skullcap. Although the device cannot predict whether an individual has a concussion or not, it can provide data in a color-coded range based on the impact or hit. The corresponding analytics program translates the color to a numerical scale (1–100) and breaks the numerical range into three color groups (green, yellow, and red) to detail the severity. Utilizing an Android or iPhone operating system platform, the user (i.e., team physician) can see where on the head the athlete was hit, the time the impact occurred, and the impact force.
In contrast to the Linx IAS wearable device, which is intended to measure the impact forces on the head, the Q-Collar device seeks to minimize the occurrence of concussions and serves as a preventative device. Specifically, the Q-Collar wraps around the neck and places slight pressure on the jugular vein (the spot on the neck where the pulse is taken). Pressure by the device compresses the jugular vein, which slows down the flow of blood leaving the brain, causing an extra 3–5 cm^3 of blood to fill up the empty spaces [9]. Because the brain is essentially floating inside the skull, maximizing the blood volume inside the brain mildly increases intracranial pressure to prevent the brain from reverberating back and forth.
In short, wearable devices show great promise to eventually reduce the incidence of concussions in athletes.
Wearable Devices for Anterior Cruciate Ligament Injury Mitigation
The anterior cruciate ligament (ACL) is the primary stabilizing knee ligament, which prevents anterior translation of the tibia. ACL tears can be broken down into two categories, contact and noncontact, which make up 30 and 70% of ACL injuries, respectively. The average recovery time for an athlete with an ACL tear is approximately nine months, and such an injury often forces the individual to miss the duration of his or her season. Currently, there is no quantifiable method to determine ACL strain and no system that accurately quantifies ACL strain for an individual at any given time. The development of a wearable sensor capable of such measurement is highly desirable in cutting and pivoting sports, such as soccer, football, basketball, and rugby.
Such a device could be designed and fabricated using piezoelectric actuation by translating the stress exerted on the ligament into a voltage or current signal capable of being relayed via Bluetooth to a portable device. Analogous to the color-coded information provided by the Linx IAS device, one can foresee such a device portraying three colors indicating the severity of impact on the ligament over time. Based on this, appropriate action can be taken by medical personnel to potentially avoid, eliminate, or decrease the incidence of these injuries among athletes.
Future Opportunities
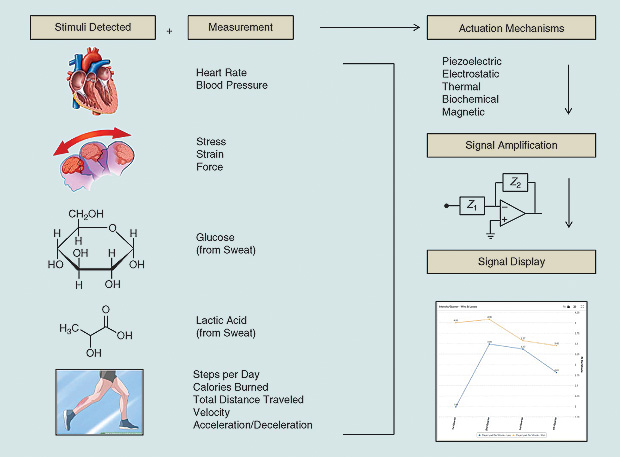
Wearable devices already demonstrate success and hold great promise in minimizing sports-related injuries and helping provide a thorough recovery and training platform for athletes. The development of an all-encompassing device capable of measuring both biometric signs (stress, strain, and impact forces) and biovital signs (glucose and lactic acid) is needed and can serve as the next gold standard for this technology. Figure 3 summarizes the wearable device pathway from detection to signal amplification for biomarkers and biovitals. The exciting opportunities merging wearable technology with data analytics stand as the next frontier for sports medicine.
References
- C. L. Dellaserra, Y. Gao, and L. Ransdell, “Use of integrated technology in team sports: A review of opportunities, challenges, and future directions for athletes,” J. Strength and Conditioning Res., vol. 28, no. 2, pp. 556–573, Feb. 2014.
- R. Li, S. R. Kling, M. J. Salata, S. A. Cupp, J. Sheehan, and J. E. Voos, “Wearable performance devices in sports medicine,” Sports Health, vol. 8, no. 1, pp. 74–78, Jan. 2016.
- R. Chamber, T. J. Gabbett, M. H. Cole, and A. Beard, “The use of wearable microsensors to quantify sport-specific movements,” Sports Med., vol. 45, pp. 1065–1081, Apr. 2015.
- T. Haberstroh. (2016, Apr.) Why the NBA slapped the wrist of Matthew Dellavedova. ESPN. [Online].
- J. Heikenfeld, “Bioanalytical devices: Technological leap for sweat sensing,” Nature, vol. 529, pp. 475–476, Jan. 2016.
- Z. Sonner, E. Wilder, J. Heikenfeld, G. Kasting, F. Beyette, D. Swaile, F. Sherman, J. Joyce, N. Kelley-Loughnane, and R. Naik, “The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications,” Biomicrofluidics, vol. 9, p. 031301, May 2015.
- A. Bandodkar, D. Molinnus, O. Mirza, T. Guinovart, J. R. Windmiller, G. Valdés-Ramírez, F. J. Andrade, M. J. Schöning, and J. Wang, “Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring,” Biosensors Bioelectronics, vol. 54, pp. 603–609, Apr. 2014.
- M. Amjadi, K. K. Kyung, I. Park, and M. Sitti, “Stretchable, skinmountable, and wearable strain sensors and their potential applications: A review,” Adv. Funct. Mater., vol. 26, pp. 1678–1698, Feb. 2016.
- S. Larson. (2015, Jan.). A new wearable wants to prevent concussions. Daily Dot. [Online].
- R. Mannix, R. W. P. Meehan, III, and A. Pascual-Leone, “Sportsrelated concussions—media, science and policy,” Nat. Rev. Neurol., vol. 12, pp. 486–490, Aug. 2016.



