Most of our everyday experiences arise through interaction with our environment as well as through socialization with people; be they loved ones, friends, or colleagues. Being able to experience and recognize sensations is critical for effective functioning and communication. Arguably, the primary sense needed for such interaction is that of vision, being the most complex and feature-rich of the senses. Vision impairment results in momentous personal and economic burdens to individuals and to society.
In developed nations, studies have shown that approximately 30% of all cases of blindness in people aged 29-40 are caused by retinitis pigmentosa (RP) (1). This disease affects approximately 1.5 million people worldwide, equating to 1 in 4000 people. RP is a degenerative retinal disorder, which begins with the death of the light-sensitive cells of the retina (photoreceptors) in the periphery, and progresses at varying rates towards the areas of the retina responsible for detailed central color vision. RP ultimately leads to profound visual impairment. There is no known effective treatment for RP. For those affected, a visual prosthesis or ‘bionic eye’ may offer significant hope and benefit. In this article we discuss the basic premise of a bionic eye, the work of our group (and briefly of others) in the development of such a device, and some crystal-ball gazing into the future of such sensory device therapeutics.
A number of possible implantation sites that interface with various locations in the visual pathway from eye to brain have been proposed for a visual prosthesis. These include the visual cortex, the lateral geniculate nucleus, the optic nerve, and various locations proximal to the retina. Retinal prostheses are the most common approach and have been implanted in patients epiretinally (2), subretinally (3, 4), suprachoroidally (5), and intra-sclerally (6). Internationally, two devices have gained regulatory approvals with Second Sight from the United States having FDA and European CE approval for an epiretinal device and Retina Implant AG from Germany having CE marking for subretinal implants.
A Suprachoroidal Bionic Eye
Our work in the area of bionic eyes has focused on a suprachoroidal implant, with recent research efforts made possible in large part through a generously-funded Australian Research Council Special Research Initiative (SRI) in Bionic Vision Science and Technology. One of two recipient teams of SRI funding was Bionic Vision Australia (BVA), a consortium of research teams in Australia of which we were a part from 2010-2015. (The Bionic Vision Australia consortium consists of the University of New South Wales, the University of Melbourne, the Centre for Eye Research Australia, the Bionics Institute and NICTA. Supporting participants are the Royal Victorian Eye and Ear Hospital, the National Vision Research Institute of Australia and the University of Western Sydney. The BVA consortium was funded between 2010 and 2015 by the Australian Research Council, through its Special Research Initiative in Bionic Vision Science and Technology Program.) Over this time, BVA developed a number of devices including a 24-channel prototype prosthesis comprising an electrode array with a percutaneous connector on the subject’s skull that could be plugged into external electronics for psychophysics experiments. This prototype was implanted in three participants in an 18 month pilot study (5). The work demonstrated proof of principle of how such a device can evoke consistent and meaningful spots of light or so called ‘phosphenes’ in response to electrical stimulation delivered from the suprachoroidal space (7).
The suprachoridal space lies between the fibrous outer shell of the eye—the sclera, and a layer of vasculature called the choroid. A key advantage of placing a device in the suprachoroidal space is that the electrodes do not directly contact the extraordinarily delicate neural retina. Furthermore, the surgical technique is rapid with no requirement to enter the vitreous cavity at all, negating the need for corneal incisions, intraocular lens extraction, vitrectomy and retinal surgery. The electrode array component of the device is instead slid into the natural cleavage plane of the suprachoroidal space via a scleral incision and fixed into position with permanent sutures.
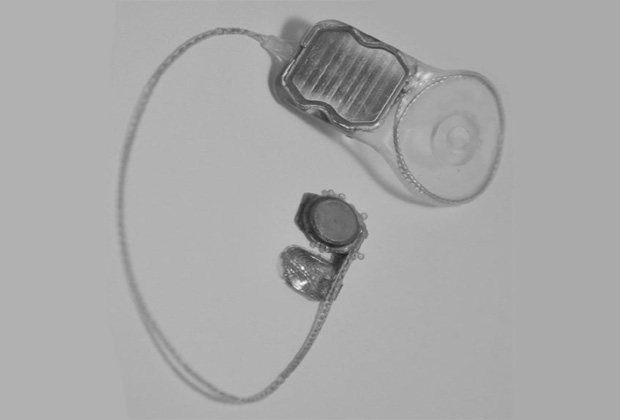
Current work (Figure 1) as described below builds on the knowledge of the pilot patient trial and is focused on a fully implanted 99-channel suprachoroidal prosthesis (Phoenix99) incorporating a novel split architecture design (8). A power and data transfer unit is implanted behind the ear in a position similar to a cochlear implant. A charge-balanced, two-wire interface connects this unit to a small-scale intra-ocular unit that is surgically attached to the outside of the globe with the electrode array of this unit being introduced in to the suprachoroidal space. The intra-ocular unit comprises solely a custom-designed application specific integrated circuit (ASIC) with no other discrete components. The Phoenix99 advances the state-of-the-art of bionic eyes through incorporation of stimulation features that are designed to provide more focused and meaningful visual perception.
In order to arrange electrodes into a dense cluster, electrodes of the Phoenix99 are arranged in a hexagonal mosaic. Clusters of seven electrodes take the form of a hexagon where six electrodes at the corners of the hexagon form a ring that surrounds one stimulating electrode at the hexagon’s center. Stimulation current delivered via the central electrode is returned via the ring of electrodes, each carrying approximately one sixth of the stimulating current. The hexagonal configuration is remarkably effective in confining stimulation to a small area of retinal tissue (9, 10), and significantly limits ‘cross-talk’ between neighboring stimulation sites. This approach allows the visual scene to be conveyed more rapidly than with series stimulation, and we believe that it may lead to more detailed vision than is possible with alternative approaches being utilized today.
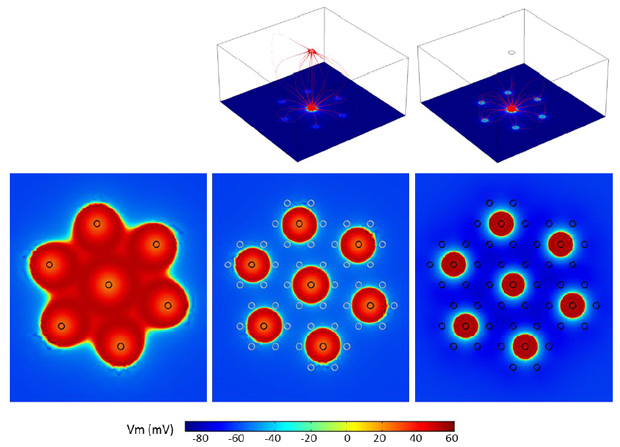
A drawback to the suprachoroidal placement is that the distance between the stimulating electrodes and the targeted retinal neurons is relatively large. Accordingly, stimulation thresholds are known to be higher in hexapolar stimulation than so-called monopolar stimulation where stimulation is delivered in reference to an electrode placed far away from the retina. We address this issue (Figure 2) by combining the threshold-reducing attributes of monopolar stimulation in conjunction with the hexapolar stimulation strategy in what we describe as ‘quasi-monopolar’ stimulation (QMP). Monopolar and hexapolar stimulation are delivered to the same stimulating electrode at the same time. Sub-threshold, monopolar stimulation serves to increase the propensity of the retinal tissue to be activated by a concomitant hexapolar stimulation of significantly lower amplitude relative to hexapolar stimulation by itself. This results in an important and significant reduction in stimulation threshold without losing the stimulation focusing benefits from the hexapolar stimulation (11-13).
A Vision for the Future – It’s All about the Interface
Casting back to the 15th century, a philosopher of the time, Desiderius Erasmus, claimed ‘in the land of the blind, the one-eyed man is king’. The popular social commentator, Marshall McLuhan, derided the view of Erasmus, postulating that ‘in the land of the blind, the one-eyed man is a hallucinating idiot…for he sees what no one else does: things that, to everyone else, are not there’. In a parallel manner, there are healthy and protracted discussions in the visual prosthesis and neural interfacing communities about number of effective electrodes needed to provide a useful device, and around the naturalness or otherwise of the perceptions evoked. Not surprisingly the interpretation of the words ‘useful’ and ‘natural’ are central to the debate.
The primary reason the debate exists is that there is a vast disparity between the technology and the biology in terms of communications channels. Whereas current bionic eyes that have been designed to last the lifetime of the patient (i.e., using hermetic encapsulation of electronics) deploy a very restricted number of electrodes (in the order of 100), natural vision involves approximately one million retinal ganglion cells (RGCs) and tens of millions photosensitive cells called photoreceptors. Following the loss of the photosensitive cells, the RGCs remain largely functional and therefore are our targets for electric stimulation of the retina. RGC axons collectively form the optic nerve, and serve as the avenue through which visual information is conveyed. Retinal visual prostheses rely upon this connection.
The mismatch between technology and biology at the neural interface is a constraint not only for bionic eye devices. It applies to all neuroprosthetics. For example, while there exist tens of thousands of spiral ganglia in the human cochlea, bionic ear technology even after three decades of development and patient feedback, remains limited to tens of electrodes—albeit with remarkable, life-changing efficacy. Looking generations forward in neural prostheses, a major aspiration for the neural interface is to approach one-to-one connections between electrodes and neurons such that stimulation can be delivered or responses recorded from individual neural cells. Despite many attempts by researchers worldwide, both the scaling up of single channels to high channel count multi-electrode arrays (MEAs) and the translation from laboratory investigations to functional commercial technologies remain elusive.
Advancement of the functional benefits that bionic eyes can provide will hopefully occur through both evolution and revolution. In terms of evolution, we are seeing more effective charge delivery mechanisms through the use of new materials and electrode coatings including conductive polymers. We also see many reports on more effective stimulus delivery and response localization through neuromodulation techniques and current steering. With the current technology and these advances, bionic eyes will see a significant improvement in functionality over the next five years. But in terms of biomimicry they will remain a poor substitute for the system Mother Nature has provided. That will require a revolution in design approaches by way of a range of disruptive technologies.
In terms of revolution or transformational changes, our vision for the next generation of bionic eyes will be realized when two conditions are met. The technology of today fails in one or both of these two conditions when electrode numbers grow to more than approximately one hundred.
The first condition that researchers and developers struggle to meet is the requirement that each electrode within an MEA must be able to communicate with the outside world in a reliable and unobtrusive means. While it may seem mundane and readily solvable, the issues of wiring, hermetic encapsulation and wireless connectivity remain a major barrier to progress in the field. Advances in the overall fields of electrical and biomedical engineering will drive device miniaturization and wireless communications technology that will support these advances.
For the second condition, neural engineering approaches must be discovered that allow for intimate, biostable and robust connections between an electrode and a small number of neurons in a manner that is scalable to individually interface with the vast number of RGCs in the retina. We all have seen and heard reports in the popular media about how this has purportedly been achieved. Similarly there are literature reports dating back decades that describe how individual cells can interface with micro-electrodes for recording and stimulation. But in the vast majority of reports when considering small electrodes in the order of tens to hundreds of microns diameter, the neural connections are not designed to work in devices implanted for the lifetime of the patient. Some exciting possibilities for next generation neural interfaces include (but are certainly not limited to): the use of soft and conformable electrode arrays and bioelectronics; living electrodes that can directly form and sustain synaptic connections with neural tissue; directed gene therapy under the electrode for creating neuronal processes between electrode and tissue; and optogenetic approaches for incorporating rhodopsins to transduce light signals into other cell types in the surviving retina and creating neural interfaces with these cells through optical technologies.
Be it an evolution in technologies, or a disruptive revolution, progress will only be seen through multidisciplinary expertise and collaboration, with biomedical engineers providing the core enabling skills in re-engineering the neural interface between technology and retina—shaping the research effort and creating a bright future for the next generation of bionic eyes.
References
- Buch H, Vinding T, La Cour M, Appleyard M, Jensen GB, Nielsen NV. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology. 2004;111(1):53-61.
- Humayun MS, Dorn JD, da Cruz L, Dagnelie G, Sahel JA, Stanga PE, et al. Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology. 2012;119(4):779-88.
- Stingl K, Bartz-Schmidt KU, Besch D, Braun A, Bruckmann A, Gekeler F, et al. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc Biol Sci. 2013;280(1757):20130077.
- Palanker D, Vankov A, Huie P, Baccus S. Design of a high-resolution optoelectronic retinal prosthesis. J Neural Eng. 2005;2(1):S105-20.
- Ayton LN, Blamey PJ, Guymer RH, Luu CD, Nayagam DAX, Sinclair NC, et al. First-in-human trial of a novel suprachoroidal retinal prosthesis. PLOS ONE. 2014;9(12):e115239.
- Fujikado T, Kamei M, Sakaguchi H, Kanda H, Morimoto T, Ikuno Y, et al. Clinical trial of chronic implantation of suprachroidal-transretinal stimulation system for retinal prosthesis. Sensors and Materials. 2012;24(4):181-7.
- Shivdasani MN, Sinclair NC, Dimitrov PN, Varsamidis M, Ayton LN, Luu CD, et al. Factors affecting perceptual thresholds in a suprachoroidal retinal prosthesis. Invest Ophthalmol Vis Sci. 2014;55(10):6467-81.
- Suaning GJ, Lovell NH, Lehmann T, editors. Neuromodulation of the retina from the suprachoroidal space: The Phoenix 99 implant. Biomedical Circuits and Systems Conference (BioCAS), 2014 IEEE; 2014: IEEE.
- Suaning GJ, Hallum LE, Preston P, Lovell NH. An efficient multiplexing method for addressing large numbers of electrodes in a visual neuroprosthesis. Conf Proc IEEE Eng Med Biol Soc. 2004;2:4165-8.
- Lovell NH, Dokos S, Cheng E, Suaning GJ. Simulation of parallel current injection for use in a vision prosthesis. Conf Proc IEEE Eng Med Biol Soc. 2005:458-61.
- Matteucci PB, Chen SC, Tsai D, Dodds CW, Dokos S, Morley JW, et al. Current steering in retinal stimulation via a quasimonopolar stimulation paradigm. Invest Ophthalmol Vis Sci. 2013;54(6):4307-20.
- Abramian M, Lovell NH, Habib A, Morley JW, Suaning GJ, Dokos S. Quasi-monopolar electrical stimulation of the retina: a computational modelling study. J Neural Eng. 2014;11(2):025002.
- Habib AG, Cameron MA, Suaning GJ, Lovell NH, Morley JW. Spatially restricted electrical activation of retinal ganglion cells in the rabbit retina by hexapolar electrode return configuration. J Neural Eng. 2013;10(3):036013.



