New approaches combine both technologies for easier and targeted drug delivery, while providing access to drug-resistant cancerous tumors and across the blood–brain barrier
Microbubbles and ultrasound are no longer teaming up only as a way to enhance images. New technologies are now using the two to create physical pathways into cells for easier drug delivery, even into the cells of highly drug-resistant cancerous tumors and across the blood–brain barrier. Going further, a Texas research group has developed drug-carrying microbubbles that can complete targeted delivery themselves, and another group in Missouri has shown that two-way traffic in channels across the blood–brain barrier also allow biomarkers to flow out, which provides a new window into the brain as well as brain diseases.
First used to boost ultrasound imaging around six decades ago, microbubbles are injected into a patient’s blood, ultrasound waves are applied at the area to be imaged, and the microbubbles resonate and reflect or echo back ultrasound waves, resulting in a better overall image. The strategy was later extended to provide other clinically important information, such as blood flow rate.
The shift to drug delivery came with the realization that targeted ultrasound can also vibrate the microbubbles in such a way that they cause transient and nonlethal pores to form in cells. Called sonoporation, this then allows a channel through which drugs can enter cells, a particularly beneficial advance for improved delivery of cancer drugs to hard-to-reach or difficult-to-permeate tumors.
Into solid tumors
Studies are already showing how this technology is aiding in the delivery of cancer drugs, even in especially difficult tumors. In December 2022, for instance, researchers at the Translational Genomics Research Institute (TGen), Phoenix, AZ, USA, (part of City of Hope headquartered in Duarte, CA, USA) reported that a microbubble ultrasound technology appreciably improved antitumor efficacy of standard chemotherapy drugs against the notoriously difficult-to-treat pancreatic cancer.
This represents a significant advance, according to the study’s senior author Haiyong Han, Ph.D., TGen professor of molecular medicine and head of the Pancreatic Cancer Program’s basic research unit (Figure 1). “Pancreatic tumors are very fibrotic, and that causes several different characteristics. One is that the vessels in the tumor are highly compressed, and another is they are fewer in number than in other tumor types, (and) since drugs move through the blood, they cannot get into the tumor,” he said. In addition, infiltration of T-cells is also lessened, which dampens a popular cancer immunotherapy known as immune checkpoint inhibitors (e.g., PD-1, PD-L1, and CTLA-4 inhibitors). Although many research groups are working on ways to resolve those issues, treatment of pancreatic tumors has remained difficult, he said, but the results of their new study are a reason for hope.

Figure 1. Haiyong Han, Ph.D., professor of molecular medicine and head of the Pancreatic Cancer Program’s basic research unit at TGen, was the senior author of a study showing that a microbubble ultrasound technology significantly improved the efficacy of standard chemotherapy drugs (nab-paclitaxel plus gemcitabine or liposomal irinotecan) against difficult-to-treat pancreatic cancer. (Photo courtesy of TGen.)
The study used a version of the technology called acoustic cluster therapy (ACT), developed by the company EXACT Therapeutics (EXACT-Tx), Oslo, Norway, and tested it on a patient-derived xenograft model of pancreatic cancer that was implanted in mice. ACT introduces both negatively charged microbubbles and positively charged microdroplets into the blood. When high-frequency ultrasound is applied, the microbubbles oscillate, which induces the microdroplets to undergo a phase shift to gas, resulting in the formation of large bubbles (approximately 22 μm in diameter). Once the large bubbles are lodged in the microvasculature of the tumor, low-frequency ultrasound is applied to oscillate the large bubbles, which in turn opens up pores in the vessels and allows the drugs to permeate the tumor tissue. The study demonstrated that the technology resulted in 7.2 times higher probability of complete remission [1] when compared to chemotherapy treatment alone.
“We are now going to test checkpoint inhibitors in an immune-competent model for pancreatic cancer (murine KPC), which has been shown to recapitulate the human pancreatic tumor microenvironment and the fibrosis, and provides an intact immune system where these transgenic murine pancreatic tumors can develop and grow,” Han said. “We hope to begin that study soon.”
In late December, EXACT-Tx announced a 16 million Norwegian krone (NOK) (about $1.5 million USD) grant from the Norwegian Research Council to support additional work on the ACT technology [2]. The grant will fund a collaboration between EXACT-Tx, TGen, the Norwegian University of Science and Technology, and Institute of Cancer Research/Royal Marsden Hospital in the U.K. to conduct six preclinical studies of ACT with checkpoint inhibitors for the treatment of not only pancreatic cancer, but also colon and breast cancer. EXACT CEO Per Walday, Ph.D., called the grant “an essential trigger for our exploration of the potential of ACT within immuno-oncology.”
On-bubble delivery
If ultrasound and microbubbles could open channels to allow systemically administered drugs into cancer cells, could the technology do more? That question led a a Texas research group to develop microbubbles that not only create pores in immune cells, but also do the job of carrying and delivering the cancer-treating cargo themselves.
“How we got interested in this area is a story of chance,” said Wen Jiang, M.D., Ph.D., assistant professor of radiation oncology and physician-scientist at the University of Texas (UT), MD Anderson Cancer Center, Houston, TX, USA (Figure 2). Jiang had been focusing his research efforts on improving cancer treatments, while chemist Jacques Lux, Ph.D., an associate professor of radiology at UT Southwestern Medical Center, Dallas, TX, USA, had been working on modifications to the surface of microbubbles with the hope of using them to convey DNA for gene therapy. “There were many advantages to ultrasound-microbubble technology: microbubbles were biocompatible, ultrasound machines were already in both hospitals and outpatient clinics, and the microbubble-ultrasound platform was already approved by the U.S. Food and Drug Administration (FDA). And it seemed to us that, if this platform could deliver DNA, then it might be possible to use it to deliver small nucleotides, including cyclic dinucleotides for cancer immunotherapy.”

Figure 2. The research groups of Wen Jiang (shown here), M.D., Ph.D., assistant professor of radiation oncology and physician-scientist at the UT MD Anderson Cancer Center, and Jacques Lux, Ph.D., associate professor of radiology at UT Southwestern Medical Center, are studying sonoporation and its potential to both carry and deliver drugs to hard-to-treat solid tumors. (Photo courtesy of Wen Jiang.)
The research groups of Lux and Jiang joined forces to develop an ultrasound-microbubble technology that would remove a common roadblock to an otherwise-effective cancer-fighting pathway. Called the cytosolic innate immune sensor cyclic guanosine monophosphate–adenosine monophosphate (cGAMP) synthase-stimulator of interferon genes, or cGAS-STING pathway, it requires activation by a cyclic dinucleotide, which the body’s immune system produces. To work, however, cyclic dinucleotide has to make its way into the cytosol of the cancer cell, and cancer cells have defenses to impede that movement. That is where the microbubbles came into play.
For their platform, which they named MUSIC for “microbubble-assisted ultrasound-guided immunotherapy of cancer,” Dr. Lux’ lab designed microbubbles that can carry both macrophage-targeting antibodies and cGAMP [3]. Microbubbles could be easily administered directly into the tumor under ultrasound guidance, because microbubbles are clinically used ultrasound contrast agents. After injection, increasing ultrasound intensity caused the inertial cavitation of the microbubbles. Their forcible collapse in close proximity to the targeted macrophages led to the transient opening of the cells’ membrane, a phenomenon known as sonoporation, and the delivery of cGAMP into the cell’s cytosol to activate the STING pathway (Figure 3). “Because this is on-demand activation, the microbubbles won’t release their cargo until enough ultrasound energy is applied, and at the same time, ultrasound allows you to image, so you know when the microbubbles have arrived at the tumor site and also when they have burst,” Jiang said.
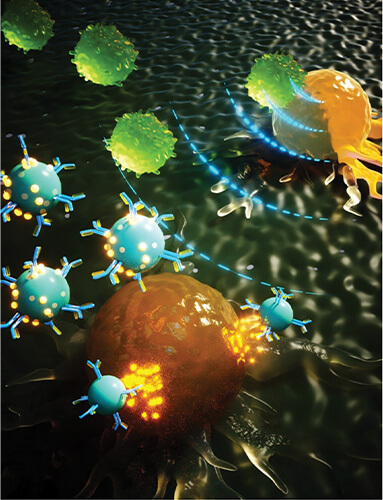
Figure 3. The UT researchers developed microbubbles (blue) that carry antibodies as well as a compound called cGAMP that activates the antitumor pathway known as STING. The antibodies help direct the microbubbles to the tumor, and once there, ultrasound (indicated by blue dashed lines) causes the microbubbles to oscillate and open transient channels or pores into the tumor cells (orange). A different frequency of ultrasound is then applied to burst the microbubbles and release cGAMP, which travels through the pores to activate the STING pathway inside the tumor cells. (Image courtesy of Wen Jiang and Jacques Lux.)
One of the innovations involved in generating this platform was the construction of the microbubbles. “Because the core of the microbubbles is made of gas, reengineering these ultrasound contrast agents into a drug-delivery platform was achieved by surface chemistry,” he described. For that, they chemically modified a sugar polymer with a biomolecule known to stabilize nucleic acids and attached this synthetic and biocompatible polymer to the microbubble surface to form stable complexes with cGAMP, which protected it from being degraded by the body’s enzymes. Macrophage-targeting antibodies were also conjugated to the microbubble surface.
Testing of the MUSIC strategy began with cell-culture models that showed delivery of cGAMP when the microbubbles burst, and then moved into animal experiments that demonstrated significant reduction in tumor growth as well as tumor eradication in 60% of subjects (triple-negative breast cancer mouse models). “We then looked at whether we could synergize with some of the already clinically approved immunotherapies, and found that we could activate the innate immune system with cGAMP and the adaptive immune system (and its antigen-specific response) with PD-1 for an even more dramatic benefit,” Jiang said, noting that the one–two punch inhibited tumor growth in both localized and metastatic murine cancer models, and increased median survival by 76% as compared to PD-1 alone.
With the success of the strategy, the researchers have started a company (MusiQ Bio) to develop the technology further and commercialize it, while also exploring its use to treat other tumors. The hope, Jiang said, is that this targeted-delivery method will mean a lesser amount of drug will work better than much higher doses of systemically administered drugs, which will also greatly reduce any side effects. He added, “Because the drugs are attached to the bubbles, and the bubbles can home in on specific cells and tissues, this would give us the precision and selectivity to accomplish this.”
Not just drug traffic
The growing interest in microbubbles and ultrasound for drug delivery spurred yet another question: Once the channel is open, what if it allows two-way traffic? It does, according to Hong Chen, Ph.D., associate professor of BME at the McKelvey School of Engineering and of Radiation Oncology, School of Medicine, Washington University (WashU), St. Louis, MO, USA (Figure 4).

Figure 4. Hong Chen, Ph.D., associate professor of BME at the McKelvey School of Engineering and of Radiation Oncology, School of Medicine, WashU. (Photo courtesy of Hong Chen.)
Chen’s doctoral and postdoctoral research centered on understanding the biophysics behind microbubbles’ effects in blood vessels, particularly for drug delivery, but when she started her own lab at WashU, she wanted to take a bigger view. “I made it very clear that the mission of my lab is technology innovation and clinical translation, which forced the whole team, and especially me, to think outside the box,” she said. “There were so many people who had already laid the groundwork and showed we can safely, noninvasively, and transiently open up the blood–brain barrier with ultrasound and microbubbles, and we said, ‘Wait a second. Once the door is open, there may be other possibilities’.”
Their research includes focused ultrasound-enabled liquid biopsy, or sonobiopsy, for sampling materials gathered from the brain side of the blood–brain barrier. She and her group began by establishing the feasibility and safety of the technique in mice and pigs, and then shifted to looking for diagnostic biomarkers for brain cancers. It worked: Sonobiopsy could detect biomarkers of brain tumors in animal models [4].
One of the reasons her group began looking for brain tumor markers is that the markers have been very well-studied in humans, thanks to neurosurgery that makes those tissues available for analysis. “But for Alzheimer’s or any other diseases of the brain, nobody would accept doing surgery or a biopsy just to analyze for biomarkers. That means that our understanding of those diseases in humans is limited, because we haven’t had access to the brain,” Chen said. With sonobiopsy, her group was able to provide that highly sought ingress.
In January, they reported that sonobiopsy across the blood–brain barrier greatly outperformed a standard blood analysis in detecting three biomarkers associated with Alzheimer’s and other neurodegenerative diseases [5]. Their results included a 2.3-fold increase in the detection of the biomarker known as neurofilament light (NfL) protein.
At this point, the WashU sonobiopsy simply involves adding a blood draw to a procedure (Figure 5). “This was on purpose. We wanted to build on what has been working, so that we can move quicker in terms of putting the tool in neurosurgeons’ hands (and start) collecting preliminary data,” Chen said.
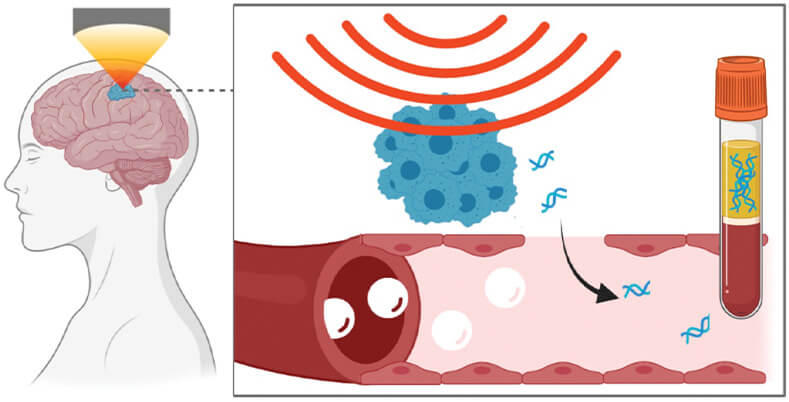
Figure 5. Chen’s research group realized an ultrasound-induced blood–brain barrier opening offers a two-way street with materials not only entering, but also exiting. That led them down several paths of inquiry, including research that showed biomarkers cross the blood–brain barrier, and can be detected via a simple blood draw. Potential applications include diagnosis of brain tumor, neurodegenerative and other brain diseases, and insights into treatment opportunities. (Image courtesy of Hong Chen.)
That preliminary data will also be coming from other research groups, she noted. “What is already happening is that multiple clinical drug-delivery studies are collecting blood now and trying to get sonobiopsy integrated into their trials, so we are going to see sonobiopsy data from multiple clinical trials very soon. That’s exactly what we wanted (Figure 6).”

Figure 6. Chen (in green shirt) stands in the middle of her large research group (17 in number as of February 2023), which includes mainly graduate students, along with several postdocs, lab technicians, and staff scientists. “This is an eye-opening experience for my students,” she remarked. “For instance, the lead author of the 2023 Radiology paper, who has now graduated, was able to see this technology move from mice to pigs to humans in just his one thesis.” (Photo courtesy of Hong Chen.)
Chen added, “If I just count on my team to move this technology forward, it will probably take many years, but if I quickly share with the community the possibility of the technology and how to do it, that’s how I think we can achieve our goal of truly impacting patient care for brain tumor and other brain diseases.”
References
- S. Ng et al., “Effect of acoustic cluster therapy (ACT) combined with chemotherapy in a patient-derived xenograft mouse model of pancreatic cancer,” J. Controlled Release, vol. 352, pp. 1134–1143, Dec. 2022, doi: 10.1016/j.jconrel.2022.11.016.
- Exact Therapeutics. (Dec. 22, 2022). Exact Therapeutics Awarded NOK 16 Million From the Norwegian Research Council for Collaboration Project With NTNU, ICR and TGen. Accessed: Feb. 27, 2023. [Online]. Available: https://www.exact-tx.com/press-releases/exact-therapeutics-awarded-nok-16-mill
- X. Li et al., “Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles,” Nature Nanotechnol., vol. 17, no. 8, pp. 891–899, Aug. 2022.
- C. P. Pacia et al., “Sonobiopsy for minimally invasive, spatiotemporally-controlled, and sensitive detection of glioblastoma-derived circulating tumor DNA,” Theranostics, vol. 12, no. 1, pp. 362–378, Jan. 2022.
- C. P. Pacia et al., “Focused ultrasound–mediated liquid biopsy in a tauopathy mouse model,” Radiology, vol. 307, no. 2, Apr. 2023, Art. no. 220869, doi: 10.1148/radiol.220869.



