Humans have been using technology to improve their vision for many decades. Eyeglasses, contact lenses, and, more recently, laser-based surgeries are commonly employed to remedy vision problems, both minor and major. But options are far fewer for those who have not seen since birth or who have reached stages of blindness in later life.
For millions of people who suffer from eye damage leading to vision loss, the promise of assistive and corrective technology to restore vision has been on the horizon for years, the true game changer soon to come. But, like many promising technologies, real breakthroughs to restore vision have faltered and stalled, even as technology to enable other senses (such as cochlear implants for hearing) have raced ahead. So why is it so hard to restore blindness?
Vision is arguably a more complex sense than hearing and presents unique challenges. For one, the eye has several layers, most notably the lens, cornea, and retina (back of the eye), each of which can incur damage that will cause vision loss. For example, retinitis pigmentosa, a genetic disease that causes a deterioration of the light-sensitive tissue at the back of the eye, leads to blindness and vision impairment as early as childhood. And age-related macular degeneration, the leading cause of blindness and vision impairment in people over 50 according to the National Eye Institute, is a result of the slow deterioration of a sensitive part of the retina called the macula, which is responsible for central vision.
While retinitis pigmentosa, macular degeneration, glaucoma, and a host of other vision-impairing eye diseases have no cure, technology finally appears on the brink of addressing the substantial impairment caused by these conditions through devices that tap directly into the eye or even the visual processing center of the brain. And advances even further off, such as light-based manipulation of cells (optogenetics) and stem cell therapies, hold promise as well.
PLugging In: Bionic Eyes
A burgeoning area of research involves restoring vision using technology in place of the diseased or dysfunctional part of the eye. In healthy eyes, rods and cones in a retina transfer light signals to electrical signals that pass through the optic nerve to the brain. Bionic eyes aim to function as the damaged part of the eye: for example, by using implanted computer chips to transform light signals into electrical signals the brain can still read or, potentially even more effective, bypassing the eye entirely and sending visually coded signals directly to the brain.
While some devices are nonsurgical, sitting on the face like a pair of glasses to present a user with amplified vision (such as those developed by Canada-based eSight 3), most are implanted into the eye, where the retina has some functionality left. A prosthetic device typically captures the images in front of a user with a camera system (either externally through eyeglasses or embedded into the implant). A computer chip then converts those images into electrical pulses that are transmitted to an electrode array implanted into the retina. The pulses stimulate what cells remain in the retina and produce a pattern of light that the brain can receive—and that the user must interpret— to regain a rudimentary sense of vision (for example, seeing general shapes move).
Several devices that have achieved functional bionic eyes are in clinical trials. France-based Pixium Vision, together with Stanford University Prof. Daniel Palanker, has created Vision Restoration Systems, a visual prosthetic; one version of this implantable medical device is being tested in clinical trials in Europe [1], [2]. A little further along is the Alpha IMS retinal prosthesis developed by Germany’s Retina Implant AG, which uses about 1,500 electrodes to interface with the brain and a built-in sensor. It recently received European approval for large-scale marketing.
Another device, the Argus II Retinal Prosthetic System developed by Second Sight based in Sylmar, California, uses approximately 60 electrodes to replace the damaged part of the retina. In addition to U.S. Food and Drug Administration (FDA) approval, the device has been authorized for use in a variety of European countries and Canada.
While these implants have been able to restore a very basic sense of vision, the next step is to achieve a more natural and realistic type of vision that allows users, even if they are completely blind, to make out details and colors. Currently, medical implants rely on the area around the retina maintaining some functionality to transmit the electric signals to the brain. If the retinal tissue is so deteriorated that this isn’t possible, researchers must bypass the eye circuitry entirely to restore vision. While researchers have tried to interface with the brain to impart a sense of vision since the 1960s, the new technology is small, biocompatible, and portable enough to be inserted into the delicate tissue of the brain’s cerebral cortex.
Bradley Greger, Ph.D., an associate professor in the School of Biological and Health Systems Engineering at Arizona State University, hopes to do just that. He focuses on neural prosthetics and neural engineering, specifically to expand on the technology of the Argus II by applying the technology directly to the visual cortex of the brain, which he speculates will lead to better vision.
“Going to the cortex would likely provide significantly better and more natural vision,” he says. “So we’ll bypass the retina altogether and implant the device directly into the brain, but still use technology that is similar to that used in the retina” (Figure 1).
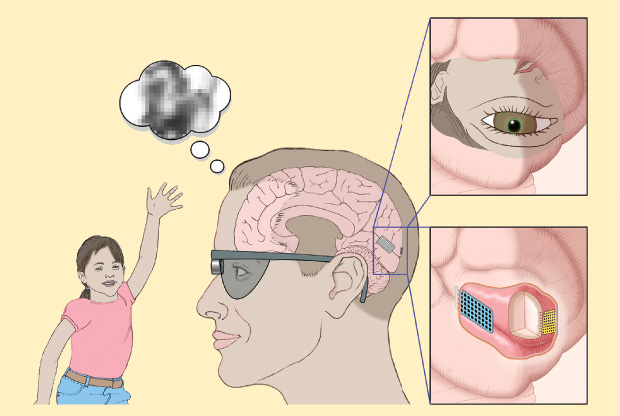
And, rather than using penetrating electrodes that go into the brain to stimulate the cortex, the new version Greger is working on would rest on the surface of the brain. “While back in the 1960s we had to use big electrodes that stimulated at high levels, now with microelectrodes I’m pretty confident we’ll be able to do it in a sophisticated manner to let people see with detail,” he says.
Vision restoration technology is about ten years behind cochlear technology but finally catching up, explains Greger. For one, surgery to put a retinal implant in the eye is more difficult; but also, even though the cortical approach predates cochlear implants, it was overhyped too early. “Everyone kept saying it would be five more years back in 1960s,” he continues. “That led to some unfortunate pushes to commercialize too early. That’s why I think the time is right now. The implantation techniques are matured, the technology is solid, so taking it into the cortex should be relatively easy.”
Greger, who spends a lot of time in the clinic with patients, says he thinks it will be two or three years before the cortical approach in human patients will restore some level of sight. “That’s what makes it exciting to go into work in the morning,” he adds. And there has been some progress with the cortical approach already: last October, Second Sight announced the first successful proof of concept for its new device, the Orion Visual Cortical Prosthetic, with a 30-year-old patient who had the system implanted on the cortex able to perceive spots of light.
While retinal implants are one of the most promising technologies coming to fruition, they themselves pose inherent challenges: the biocompatibility and long-term stability of the materials; the need for delicate, complex surgery; and the high cost of the products and procedures.
Other Technologies on the Horizon: Harnessing Light
In addition to computer chip-based implants, other efforts have looked at optogenetics, where gene therapy and light can be harnessed to manipulate cells. For example, research efforts are underway to combine gene therapy with glasses to impart a sense of vision to those who are severely blind. First, modified viral vectors— which carry genetic instructions for creating light-sensitive proteins produced by green algae—would be injected into the eye to prompt cells to become light sensitive. If the procedure works, it would not restore normal sight: rather, some cells in the eye would be light sensitive again, which could be coupled with goggles to modify the natural signal around the user. Such a clinical trial is currently underway by Retro-Sense Therapeutics, based in Ann Arbor, Michigan, and acquired by Allergan.

GenSight Biologics of Paris, France, is also planning to combine optogenetics with biomimetic goggles (Figure 2) to amplify visual information at the wavelength the cells expressing the optogenetic protein react to. The company is currently testing the safety of its gene therapy candidate and plans to move to clinical trials for retinitis pigmentosa patients later this year.

Sheila Nirenberg, a neuroscientist in the Department of Physiology and Biophysics at the Weill Medical College of Cornell University, is combining a refined computer chip with an optogenetics approach (Figure 3, right). She developed a chip for treating blindness that converts images into electrical pulses that are then sent to the brain to produce visual perceptions. “What makes this chip different from others is that it acts essentially like an artificial retina—that is, the patterns of pulses it produces when it receives an image are very similar to the patterns of pulses a normal retina would produce, and this is true for images of faces, landscapes, people walking, and other dynamic scenes,” says Nirenberg, who published the initial work in animal studies in Proceedings of the National Academy of Sciences in 2012. She’s recently converted the chip into a prosthetic device for patients (it interacts with the eye through optogenetics), and her group is aiming to secure approval from the FDA for the first clinical trial.
Nirenberg founded a startup called Bionic Sight to develop the treatment, and in January 2017 the company announced a partnership with Applied Genetic Technologies Corp. (AGTC), which develops adeno-associated, virus-based gene therapies. AGTC will develop a vector to deliver the optogenetic protein to the eye, and Bionic Sight will provide the artificial retina-based device, which ultimately will be worn in a pair of eyeglasses, according to Nirenberg. The two companies hope to restore a significant amount of sight to patients with retinal degenerative diseases.
“I think the optogenetics approach has enormous potential in the future,” adds Greger. Because it’s more complex biotechnology combined with biomedical devices, however, he says it will be a long road to get to approved products. But once it does, “it will be a real game changer.”
A Window into Stem Cell Therapy

Stem cell therapy is another promising avenue, although likely further in the future. These attempts generally aim to introduce cells that have stem cell-like properties—in essence, the ability to regenerate tissues to regrow structures, such as the eye or other organs. Researchers across the globe have made some progress in generating new tissues using stem cell methods, but the technology is far from being clinically approved in humans. Nevertheless, the approach holds potential for one day restoring vision.
Jannick Rolland, the Brian J. Thompson Professor of Optical Engineering at the University of Rochester’s Center for Visual Science as well as director of the R.E. Hopkins Center for Optical Design and the National Science Foundation Center for Freeform Optics there, is looking at potential cell regeneration in the eye tissue of a particular area that most prosthetics can’t help: the cornea (Figure 4, right).
“The cornea is the window to the world,” says Rolland. Corneal grafts are one remedy for damage, but the procedure is difficult and expensive and also requires a donor. “We’re seeing if maybe there is something else we can do, which is to stimulate implanted or naturally occurring stem cells for cell regeneration, if you can detect early on a problem. But first you need the technology to be able to look into the eye.”
Her team developed a high-definition imaging approach, Gabor domain optical coherence microscopy, to identify and (with new features) track cells to a higher degree than traditional approaches. It images cellular structures within the eye to more accurately see corneal disease by counting the death of endothelial cells. Because these cells lie on a curve (the eyeball), the technology must capture the volume of the space, flatten it, and count the cells, not an easy computational task (Figure 5).
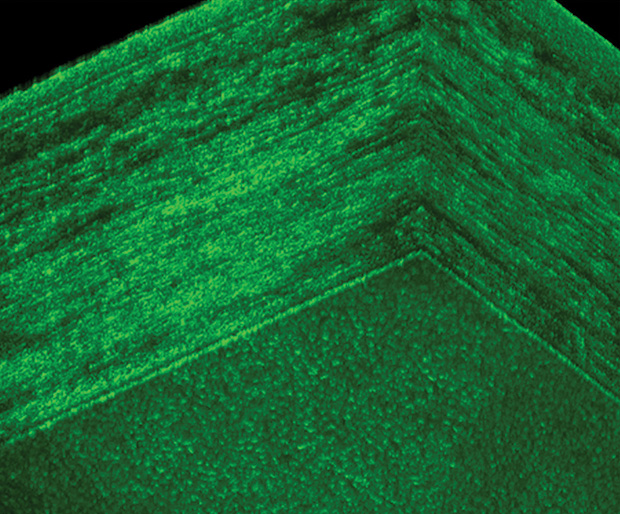
Rolland commercialized this technique by cofounding a Rochester-based company, LighTop- Tech. “This is a high-definition optical imaging technique that allows you to look at imaging the cornea in a larger field of view than current techniques,” she says. She partnered with Amy Kiernan, an associate professor at the University of Rochester, to use this more accurate recording of corneal cells as a way to trace naturally occurring potential stem cells that could be stimulated to migrate to the cornea and repair damage in mouse models.
“This is very exciting technology that has the potential to reveal inherent repair mechanisms in the cornea, although of course these studies are still at the beginning stages,” explains Kiernan.
As more retinal prosthetics prepare to come to market and as other techniques in the lab are explored, there are limited but helpful technologies more widely available now. In one approach, some patients have had success restoring vision after a stroke or brain injury by using computer-based light programs to exercise their eyes. So-called vision restorative therapy techniques use computer-based light programs to essentially coach people with failing vision to improve their sight with repetitive light stimulation; such stimulation activates areas of the vision that have suffered some injury but can still be retrained. NovaVision in Boca Raton, Florida, is one such company, offering an FDA-approved vision restoration therapy through an Internet computer-based tool called NeuroEyeCoach that trains patients to move their eyes to maximize their remaining visual field. Many other technologies also exist to help boost sight for those who are vision impaired, including assistive glasses, canes, and magnifiers [3].
Although game-changing technologies to fully restore sight have hovered just beyond reach of vision researchers, it seems that some significant strides have been made. If the pace of research continues, before long, patients who have struggled with blindness may finally get the opportunity to see.
References
- K. Grifantini. (2016, Jan.). Star Trek in real life: How close are we? IEEE Pulse. [Online]. 7(1).
- N. Lovell and G. J. Suaning. (2016, Feb. 19). Visions of a bionic eye. IEEE Pulse. [Online].
- L. Mertz. (2016, May 23). A better view: New low-vision technology helps bring the world into focus. IEEE Pulse. [Online].



