Around 2008, endoscopists David Carr-Locke and Petros Benias began to notice an unfamiliar pattern in the bile duct during endomicroscopy, which didn’t look like anything they knew from pathology. Their confusion as to what it was persisted, so they brought their observations to their colleague, pathologist Neil Theise. Eventually, a larger group of researchers worked to figure out what exactly they were seeing in the bile duct samples. Their ultimate conclusion was that the structure is part of a network of connected interstitial spaces.
Their discovery is nothing less than that of an entire system that may wend its way throughout the body, from fascia to brain, through intestines and lymph nodes. So far, it appears in every place they have gone to look for it. While science has noted these extracellular spaces previously, never before had anyone conceived of them as belonging to a system in which fluid flow communicates information (Figure 1).
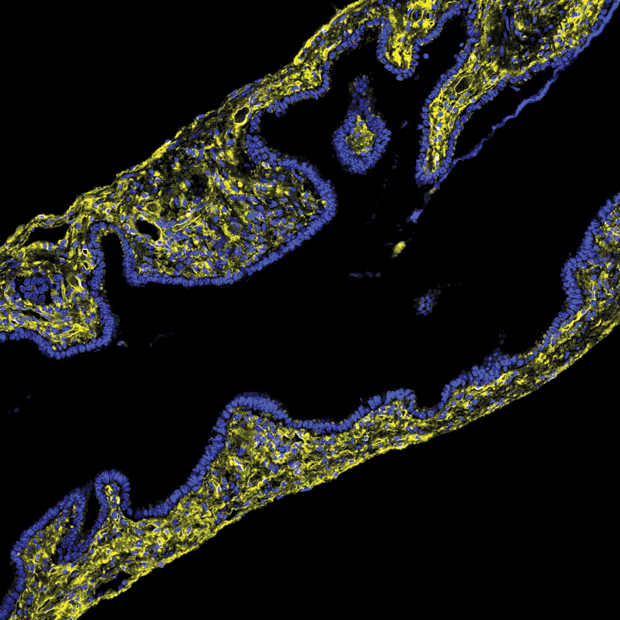
The study detailing these findings was published earlier this year in Scientific Reports, with Carr-Locke and Theise as senior authors, and Benias and Rebecca Wells, a professor at the University of Pennsylvania’s Perelman School of Medicine, as co-first authors. The report significantly expands the concept of a human interstitium, a term that had been used before but previously referred to interstitial spaces in a disjointed, limited sense. Some are even calling this new interstitium an organ. Whatever eventual label it may bear, the interstitium is now before us as a winding microscopic frontier throughout the body, with the potential of opening up new paths in disease treatment. The interstitium may be a conduit for immune cells, it may offer a blueprint for the sources of fibrosis, and—a potential showstopper—it may be a way that cancer spreads.
A Perplexing Pattern in the Bile Duct
The interstitium, pervasive but unnoticed for so long, first surfaced as a honeycomb latticework pattern with black lines through Carr-Locke’s endomicroscope. He couldn’t name it; nor could Benias, his colleague at Mount Sinai Beth Israel Medical Center. “We kept seeing the same pat- tern in the normal bile duct, which people had tried to explain in various ways, and none of which made any sense to me,” Carr-Locke recalls (Video Figure 2, below).
FIGURE 2 A video of an in vivo confocal laser endomicroscopy of a normal bile duct. (Image courtesy of Dr. David Carr-Locke.)
Seeing the honeycomb pattern coincided with another development: they were looking at the bile duct through a new type of lens, a miniaturized confocal microscope small enough to fit within a flexible endoscope. The technology allowed the doctors to focus in detail on a particular plane (like a pinhole camera does) and to do so in real time, in vivo, to see living cells—things like red blood cells flowing in small blood vessels or individual epithelial cells in the gut—in addition to this puzzling pattern in the bile duct. “We have to find out what this is,” Carr-Locke thought. “ Otherwise, we’ll be looking at this for years and calling it ‘black lines and white spaces,’ and that’s terrible. We need to know what these structures actually are.”
Diagnostic liver pathologist Theise, also at Beth Israel, had a hunch that longtime colleague Wells, a fibrosis expert, could help them test some hypotheses. Wells used second-harmonic generation imaging to visualize the bile duct samples (Figure 3). The images offered secondary proof that what they’d seen in frozen bile duct tissue samples was indeed collagen fibers. Wells remembers the moment they were looking to see how collagen in the submucosa of the bile duct was organized. “[We] saw these just beautiful, beautiful cords of collagen separated by spaces and started jumping up and down in the small, dark room.”
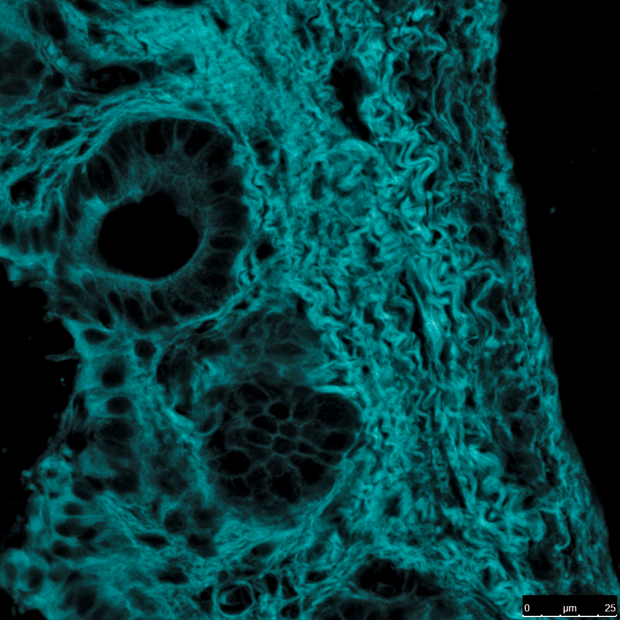
Those spaces, together with the collagen bundles, now have a collective name, the interstitium. Whether the interstitium should be classified as an organ depends on how you define “organ” and is up for debate, including by the study’s coauthors, several of whom would rather talk about what this space might contain and do, rather than how to label it just yet.
What the researchers are saying with certainty is that observation of extracellular matrix materials up to now provides only an incomplete account of the interstitial spaces in the body. They want to expand the concept of “interstitial”: “it’s not only inbetween cells,” clarifies Wells, “but within organs and between organs.” Perhaps even more significant, they say, is that those spaces are connected and the fluid flow between them makes the interstitium not merely a space but a system that communicates. Imagine, as an analogy, finding out that a small underground bunker you’ve inhabited for years connects to an extensive warren of other spaces, with intercoms in all of them. “It’s a highway of communication,” says Carr-Locke. “If it’s everywhere,” he adds, “it ends up being a huge space.”
(Finally) Seeing the Space and Its Contents
“Everywhere” so far (current research pending) includes in the bladder, skin, stomach, intestine, colon, and lungs as well as around blood vessels, ligaments, fascia, and possibly the brain. How could something found throughout the body have been hiding in plain sight? The answer is due not just to the special lens of confocal microscopy but also to the standard methods for fixing tissue samples. These use formalin and paraformaldehyde, which dehydrate tissues, meaning that the fluid contained within the interstitium disappeared during fixation. Carr-Locke and Benias were able to see that unique bile duct pattern because they were looking at living tissue that wasn’t dehydrated.
Not all tissue samples are fixed using chemicals; other samples are frozen, especially when the examination must happen during surgery. In those instances, the fluid and spaces between the collagen bundles were largely preserved but were thought to be an effect of the freezing. “People had always assumed that … when you froze tissues, for some reason you got gaps between collagen,” says Wells. Now that those gaps are known to be part of the interstitium’s structure, the work to understand what is contained in those spaces (and what other cells may pass through) is under way. These contents hold the clues to what the interstitium’s function and by-product roles in the body may be.
Collagen and a Potential Relationship to Fibrosis
To date, what has been found in the interstitium? In addition to the collagen fibers that constitute the interstitium’s support “scaffolding” and the fluid that flows through them, Wells says they also found elastin fibers and some “odd-looking cells” that are flat and resemble fibroblasts, the cells that make collagen (Figure 4). Broadly speaking, collagen is present in many types of tissues, giving them structure. One theory about the interstitium is that it has a mechanical function as a shock absorber or cushion for parts of the body that move or pulsate regularly or that are prone to impact.
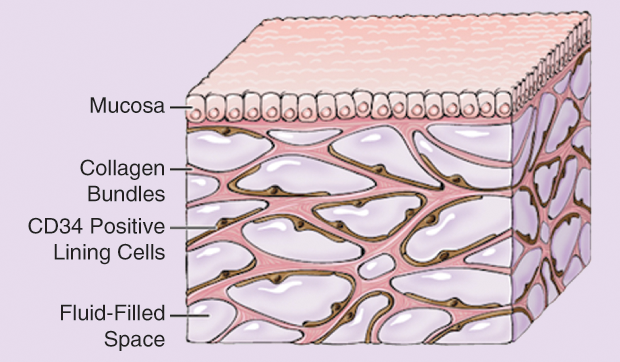
Collagen is also the building block of scar tissue, which can occur all over the body in response to disease states as well as trauma. An overproduction of fibrous tissues (e.g., collagen) can have serious consequences for the organs in which it occurs. Given collagen’s central place in the interstitium, Wells wonders if it has a relation to fibrosis. “Are cells in this space potentially cells that can cause fibrosis in some settings?” she asks. And, if so, Carr-Locke points out, “if there was some way to influence [the production of those cells], maybe those cells could be targeted with something to stop doing what they’re doing.”
Wells’s research group has spent the last two years looking into how collagen is deposited in the interstitium and what the space may look like during development. “In the neonatal mouse, there’s no collagen at birth. By day 7, there’s a large amount. Some cell has to be depositing it,” she explains. “The question is, do those ‘depositor’ cells just go away, or do they become quiescent in the adult? Then, if there’s another injury, do they reactivate and produce more collagen and fibrosis?”
A Fluid Transfer of Cells and Their Information
Learning more about the fluid that flows through this space is another research priority. So far, the fluid appears to share similar characteristics with lymph. Experiments with flourescein, an injectable dye, have shown, too, that the submucosal space (i.e., the interstitium) must connect to nearby lymph nodes, based on where the dye is injected and later appears. While there’s no proof yet, this fluid flow could be the way that the interstitium moves things from space to space in the body. “Those things might be cells, those things might be immunologically competent cells that communicate information, signals, from one place to another,” says Carr-Locke. Wells emphasizes that this is one of the major questions before us: How do immune cells move through this space and interact with it?
Answering these questions could determine the interstitium’s potential influence on immunotherapy. Until more is known about the immunological characteristics of the space, any ideas are speculative. Carr-Locke gives a hypothetical example of where they might lead: “Let’s say immunologically competent cells do pass through this space as they do in lymphatics. Lymphocytes are the obvious target. … If we can access the space in a particular organ, we could influence a disease state in that organ, by either injecting or giving something that would accumulate in that space.”
An Interstitial Spread of Cancer?
If the interstitium is a conduit within the body, akin to blood or lymphatics, then it could follow that not all the movement it enables is benign. Consider the spread of cancer. Carr-Locke describes what they’ve seen in surgeries: when part of the intestine is injected with India ink to mark it, the ink shows up in nearby lymph nodes as well. Could the interstitium be the pathway for that transfer of fluid? By the same logic, Carr-Locke says, “this may be the reason that when tumors start to penetrate into the wall of the intestine, when they get to the submucosa, which is in fact the same space that we’re talking about, that’s when the tumor can suddenly spread to the lymph nodes and everywhere else.” The interstitium could also be the unwitting conduit for cancer cells that get shed during removal of tumors in some cases, when cancer later turns up in another location.
Wells adds that a number of groups are already looking at how cancer cells metastasize along cords of collagen and through spaces that have a large number of proteoglycans. “And that’s exactly the description of this space [the interstitium] that we’re describing,” Wells affirms. Understanding how the interstitium relates to metastasis could open up a new means for treating cancer.
Bioengineering and the Interstitium
Although this expanded concept of the interstitium is still early stage, there are already indications for how bioengineers may get involved in the research. There is a design need for miniaturized devices to evaluate the interstitium without changing it and for diagnostic devices that can take very small fluid samples to analyze protein content, immune status, and cellular structure. Tissue engineers, meanwhile, will want to mimic the architecture and fluid flow through this space when engineering multilayer tissues.
For studying tumor metastasis, it will be important to include a representation of the interstitium when modeling cell behavior in tissues. Also, should the science confirm the theory that cancer spreads via the interstitium, bioengineers could help to design something to stop the spread of disease in lieu of cutting out tumor or tissue—measures that sometimes spread cancerous cells.
“For me,” says Carr-Locke, “this is perhaps the most exciting because if we could do something to that space, either locally around a particular lesion, a particular tumor, or if we could target the cells that are in that space to do something, to create a barrier so it doesn’t spread … there are all sorts of ways you could think of influencing how cancer cells can spread through that space [the interstitium] to the lymph nodes.”



