The 2018 Nobel Prize in Physics was awarded to three scientists in the field of laser science: Dr. Arthur Ashkin for his invention of the optical tweezers and their application to biological systems, and Dr. Gérard Mourou and Dr. Donna Strickland for their method of generating high-intensity, ultrashort optical pulses. The awards integrate the far reaches of time and intensity scales in laser technologies, from the extremely high-intensity chirped pulse lasers (by Mourou and Strickland) to the ultralow-power beams (by Ashkin) that are capable of handling delicate biological objects and molecules [1], [2]. The IEEE family is indeed delighted to see two of its Life Fellows, Arthur Ashkin and Gérard Mourou, as co-recipients of the awards from the Royal Swedish Academy of Science. Mourou is a past recipient of the IEEE Photonics Quantum Electronics Award and the IEEE David Sarnoff Award. Strickland has been an active author in the IEEE Journal of Quantum Electronics and IEEE Journal of Selected topics in Quantum Electronics.
The laureates
Arthur Ashkin, born on September 2, 1922, in Brooklyn, New York, is an experienced physicist and played a key role in many important discoveries in laser science before his work on optical tweezers. After studying physics at Columbia University, he went on to work in nuclear physics before turning his attention to microwave and then laser physics, working at the prestigious Bell Laboratories for a career that ended up spanning four decades. There, he began studying the concept that electromagnetic radiation, including light, could exert forces on objects. In 1969, he demonstrated that it was possible to use these minute forces to control the movements of micron-sized particles, finding that they were drawn into the center of the laser beam and were accelerated in the direction of the light [3].
At the time of the publication of this work, Ashkin did not realize the profound implications that his discovery would have for the study of biological molecules. In fact, in a telephone interview with Adam Smith, Chief Scientific Officer of Nobel Media [4], Ashkin said that at the time he thought that light was supposed to kill small living things, such as bacteria. It was only in 1986 that Ashkin and his colleagues trapped a biological object, in this case a virus in water [5]. In the process of performing these experiments, they also noticed “the appearance of some strange new particles in diluted samples that had been kept around for several days” that were moving by themselves through the water. When a microscope was used to identify these mysterious moving particles, they turned out to be bacteria from the air, which had contaminated the sample. When they wandered into the optical trap, they were caught but remained alive. Amusingly, for subsequent experiments in the same publication they used bacteria grown from pieces of a ham sandwich belonging Ashkin’s colleague [6]. While the bacteria did suffer from photo-damage from the laser, this was nevertheless the first published example of optical manipulation of living cells and paved the way for huge advances in the field of biophysics, including the possibility of observing single protein molecules folding and unfolding.

Gérard Mourou, was born in France on June 22, 1944, and studied physics there before moving to the United States, where he worked both at the University of Rochester, New York, and the University of Michigan, Ann Arbor. His work in ultrafast laser technology has contributed to developments in a number of different fields including electronics, optoelectronics, archaeology and medicine. Donna Strickland, born May 27, 1959, studied physics before joining the University of Rochester to pursue a Ph.D. in Optics. She then worked for the National Research Council of Canada, the Lawrence Livermore National Laboratory, and Princeton University before finally joining the University of Waterloo in 1997, where she has worked since then. Her research focus also is in the field of ultrafast lasers, and she has developed a number of techniques to push the boundaries of what is possible with these technologies. Strickland and Mourou were working together at the University of Rochester in the early 1980s when they developed a technique by which ultrashort pulses of very intense laser power could be generated. Their technique is now included in the design of all high intensity lasers and has enabled lasers to be used for incredibly precise and delicate operations, such as laser eye surgery (see biographies and prize shares in Figure 1).
Optical trapping and how it has changed our world
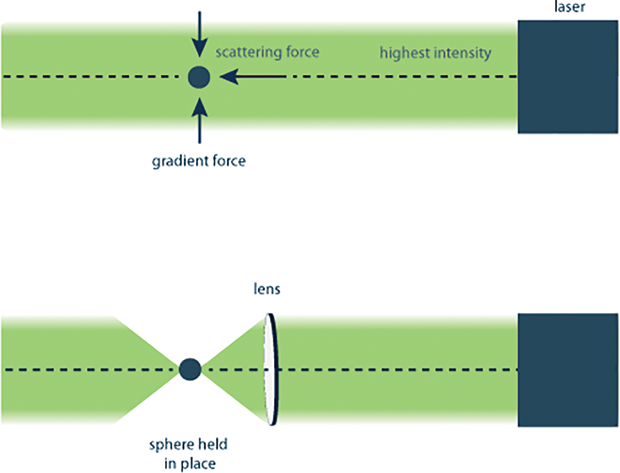
The principle of optical trapping is based on the concept of radiation pressure, also called optical force, that is, the fact that photons carry a very small momentum. Because of this momentum, they can exert small forces on objects of specific sizes. The force is exerted in two ways— by reflecting off the object (the scattering force) and by traveling through the object if it has the correct size and optical properties (the gradient force). The scattering force acts in the direction of the light, whereas the gradient force pulls the object into the area with the highest light intensity (Figure 2). The more photons there are, the larger the force exerted by them, therefore the more intense the light source the more effective the trapping. Optical trapping also depends on the size of the object being trapped relative to the wavelength, or color, of the light. Different colors of light have different wavelengths, with blue and ultraviolet light having shorter wavelengths than red and infrared light. Therefore, light sources such as lasers, where the photons are all one color, are needed to create an effective optical trap.
Optical trapping in its different forms can be used for objects on a huge variety of scales, with many existing and potential applications. The scale ranges from single atoms, as in the “optical molasses” method developed by Steven Chu, to whole cells, as already described. This technology has enabled a dazzling array of measurements to be performed. Using optical tweezers, it is possible to manipulate as well as measure the motions of individual protein motions and DNA, objects that are impossible to clearly see with even the most powerful optical microscopes (Figure 3). These results can then be used to gain a detailed understanding of how these molecules work. Recent examples include the observation of the opening and closing of two “lids” in a single enzyme molecule [7], the combination of optical tweezers with protein-engineering techniques to modify a protein that could not previously fold independently to be able to do so [8], the observation of how the enzyme responsible
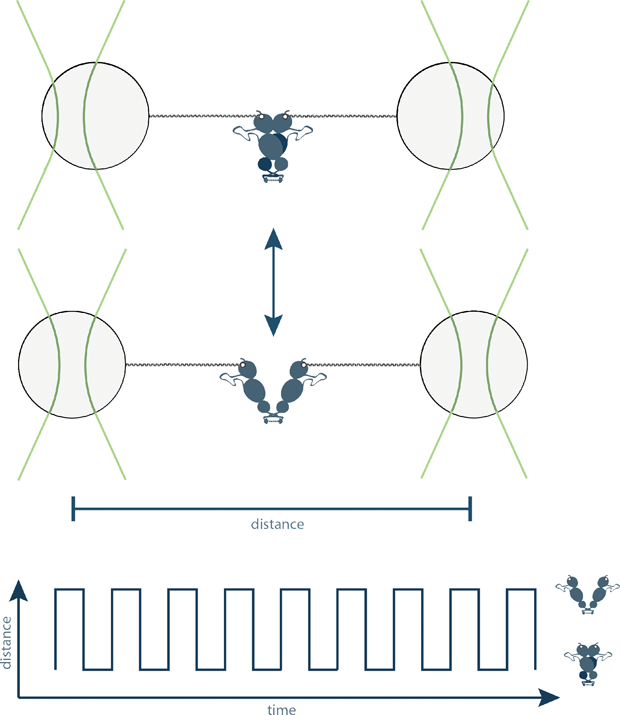
for copying a DNA sequence into an RNA sequence works [9], and a detailed comparison of proteins that do the same job in different organisms [10]. In other words, because of this technique, we are now able to see the smallest machines in our cells doing their jobs.
This is important because in doing so, we learn how they work and therefore how we can try to fix them when something goes wrong, for example, by designing therapeutic compounds that specifically modify the behavior of only one individual protein.
Ultrashort optical pulses—how they are generated and used
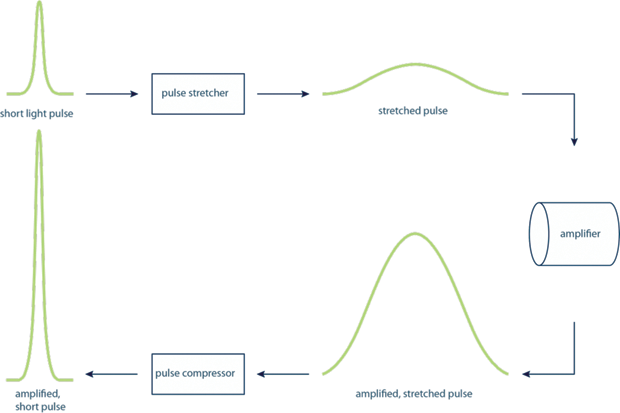
The uses and applications of lasers in science and technology are far-reaching and incredibly diverse. From manipulating the smallest molecules, as described above, to high-speed communications, spectroscopy, nuclear fusion, cutting, drilling, welding, and myriad others. For many of these applications, it is important to be able to use high-intensity laser light. However, generating laser pulses with high-power intensities was very technically challenging as it often led to the destruction of the amplifying material. The work of Mourou and Strickland resolved this issue by splitting the amplification into steps, in a technique called chirped pulse amplification (CPA) (see Figure 4). The laser pulses were first stretched in time, reducing their peak power but keeping the overall amount of power in the pulse. They were then amplified, then compressed again.
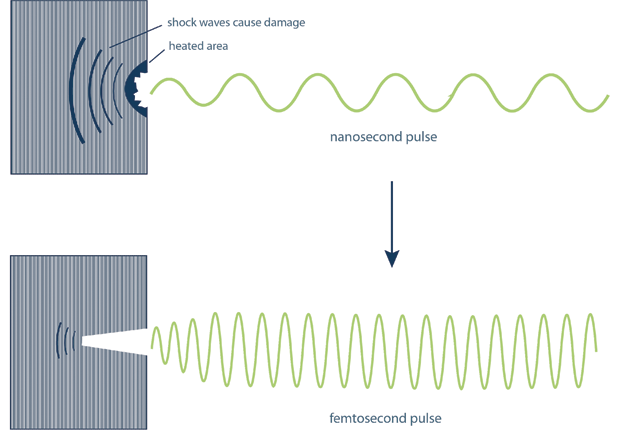
Because of the short pulse width of the generated light, pulses produced by CPA do not cause the kind of additional damage typically associated with high-power lasers (see Figure 5). For a nanosecond pulsed laser, damaging mechanical shock waves are generated in the material because of the longer duration of the pulses. These are drastically reduced for the shorter femtosecond-duration pulses generated using the method developed by Strickland and Mourou. This is why these pulses are suitable for use in delicate surgical procedures such as laser eye surgery, where minimizing the damage to the surrounding tissue is essential.
The far-reaching impact in engineering and medicine
The discoveries have a far-reaching impact beyond the tenets of basic science. One of the greatest applications of optical tweezers is in biomedical science, where researchers have effectively used them, for example, to sort healthy cells from infected ones, or to isolate cancerous cells from nonmalignant species. Over the last decades, optical tweezers slowly evolved from being manipulation tools to measurement devices, enabling precise quantification of the minute movements of trapped objects and the application of incredibly small forces to such objects. This has led to its use in the investigation of individual molecular motors, enabling previously inaccessible functional details of the motors involved in muscle function, waste disposal in cells and prevention of protein aggregation, to be studied. Karin Schütze, a biologist and coworker of Ashkin later founded Cell- Tool, a company that uses optical tweezer technology together with spectroscopy to create user-friendly tools for cancer detection [11].
Mourou and Strickland’s discovery has revolutionized laser technology. The first demonstration of CPA by the duo produced picosecond pulses delivering approximate gigawatts of power, but with technological progress, today we are able to achieve femtosecond lasers delivering terawatts of power. This unprecedented boost in laser power has not only fostered scientific developments in strong field physics and attosecond science but also opened up possibilities to test plasma acceleration of electrons and ions.
CPA has led to a plethora of new applications in chemical industry and medicine. Undoubtedly, the two principal applications of CPA are in Lasik (laser eye surgery) and in radiation therapy for oncology. In laser eye surgery, the laser ablates a selected region of the lens in the eye without damaging the neighboring tissues. It can thus be safely used for corrective eye surgery, which has benefited more than 10 million people in the United States alone.
There has been continuous progress in ophthalmological interventions using CPA. Recently, the team of Prof. Wayne Knox at Rochester has been working with Clerio Vision to develop new methods of using ultrafast lasers for noninvasive eye surgeries and to modify contact and intraocular lenses. One key benefit of these new procedures is their noninvasiveness (no cutting of the eye required), allowing multiple corrections of the lens and cornea of the same person multiple times with the progression of age.
CPA plays a significant role in radiation oncology, with several clinical centers using the technology to accelerate protons for proton therapies used to treat deep-tissue tumors, especially in the brain. The use of CPA has allowed medical physicists and clinical oncologists to deliver precise energy dosages to deep-seated tumors without damaging surrounding brain regions. This has led to unprecedented gains in quality of care and extended the posttreatment life expectancy of cancer patients. The full potential of the techniques that have been awarded Nobel Prizes has hardly been met, and now that the broader research community has an awareness of how they work, new developments in fields where the techniques have not previously been applied are likely.
Apart from their scientific discoveries, the life experiences of the laureates are no less inspiring. Ashkin provides a good lesson in perseverance by showing us what can be achieved by having an idea and ensuring that it is seen through to completion. As Michael Schirber, a Corresponding Editor for Physics, observes, “For years he worked in a minority field, pioneering and then refining his ideas, inventing techniques that scientists now use as essential tools of their trade.” Interestingly, despite being only the third woman to ever be awarded the Nobel in physics, after Marie Curie in 1903 and Maria Goeppert-Mayer in 1963, Strickland sees herself as a scientist and not a woman in science. Despite this, she will certainly be seen as a positive role model for many young women who are struggling against the gender discrimination that is so obviously present in STEM (science, technology, engineering, and mathematics) subjects.
Despite the plethora of technological developments brought about by the discoveries of optical trapping and CPA, we are only just scratching the surface of what it will be possible to do with these techniques.
References
- J. Hecht, “2018 Nobel physics prize for pioneering laser work,” IEEE Spectrum Tech Talk, Oct. 2018. Accessed: January 7, 2019. [Online]. Available: https://spectrum.ieee.org/tech-talk/semiconductors/optoelectronics/2018-nobel-physics-prize-for-laser-work.
- The Nobel Prize in Physics 2018. Accessed: January 7, 2019. [Online]. Available: https://www.nobelprize.org/prizes/physics/2018/summary/
- A. Ashkin, “Acceleration and trapping of particles by radiation pressure,” Phys. Rev. Lett., vol. 24, no. 4, pp. 156–159, Jan. 1970.
- A. Smith, “Telephone interview with Arthur Ashkin,” nobelprize.org, 2018.
- A. Ashkin and J. M. Dziedzic, “Optical trapping and manipulation of viruses and bacteria,” Science, vol. 235, no. 4795, pp. 1517–20, Mar. 1987.
- A. Ashkin, “History of optical trapping and manipulation of small-neutral particle, atoms, and molecules,” IEEE J. Sel. Topics Quantum Electron., vol. 6, no. 6, pp. 841–856, Nov. 2000.
- B. Pelz, G. Zoldak, F. Zeller, M. Zacharias, and M. Rief, “Subnanometre enzyme mechanics probed by singlemolecule force spectroscopy,” Nature Commun., vol. 7, p. 10848, Feb. 2016.
- D. Bauer et al., “A folding nucleus and minimal ATP binding domain of Hsp70 identified by single-molecule force spectroscopy.,” Proc. Nat. Acad. Sci. U.S.A., p. 201716899, Apr. 2018.
- M. Righini et al., “Full molecular trajectories of RNA polymerase at single base-pair resolution.,” Proc. Nat. Acad. Sci. U.S.A., vol. 115, no. 6, pp. 1286–1291, Jan. 2018.
- M. Jahn et al., “Folding and domain interactions of three orthologs of Hsp90 studied by single-molecule force spectroscopy,” Structure, vol. 26, no. 1, 2018.
- A. Ashkin, K. Schutze, J. M. Dziedzic, U. Euteneuer, and M. Schliwa, “Force generation of organelle transport measured in vivo by an infrared laser trap,” Nature, vol. 348, no. 6299, pp. 346–348, Nov. 1990.



