The smooth, powerful muscles of a newborn baby’s heart are pulsing normally, squeezing in and letting go rhythmically as a 3-mm-wide catheter-like tube snakes its way through, entering via an artery and being guided slowly by a surgeon. When it reaches its target—a protruding knot of malformed muscle tissue within a ventricle that has been partly blocking the valve—the tip of the precisely controlled tube whirs into action, with tiny scissor-like rotating blades gently grinding up the excess tissue as those pieces are sucked back into the device, leaving no floating particles that could lead to a blockage elsewhere. The defect is fully removed, and the heart’s function is restored to normal, leaving the child with the prospect of a normal life. The whole minimally invasive process takes place inside a beating heart and would otherwise have required open-heart surgery, with the heart stopped for a cardiopulmonary bypass.

Until now, such a device—small enough to fit inside the hearts of infants or children—did not exist, and this one, developed in the lab of Pierre Dupont (right), a bioengineer at Boston Children’s Hospital, has so far only been tested in vivo in pigs. It joins a similar tool for pulling together adjacent bits of tissue to repair holes, also developed in Dupont’s lab and tested two years ago, that could ultimately lead to a set of surgical tools that could be used in lieu of risky open-heart surgery. It is just one of many innovative ideas being developed at labs around the country that are looking at intriguing ways to address heart problems in children, despite the fact that there is little commercial incentive to do so.
Very young children and newborns suffering from severe heart malformations face what is indeed a “heartbreaking” condition, often requiring rapid and extreme interventions. Many of these children have conditions involving specific genetic mutations affecting the structure of the ventricles or heart valves. These deformations are sometimes so rare that there is limited financial return for the long years of development, testing, and approvals needed to bring a new device or treatment into clinical practice.
At this point, Dupont says, “The number of kids with any given condition is not enough to justify the commercial investment” needed to develop a significant new specialized tool for a particular need. “There may be a huge benefit, but it’s going to be a huge amount of my time, and US$1 million or more to get regulatory approval. Do I give up everything else I’m doing in my lab to do this? It’s a vexing situation, and it’s one that I bump up against all the time.”
On the other hand, tools developed for a specific pediatric condition are likely to find other applications. “If you can find a solution to help kids, it will also help adults,” he says. For example, tools that could be used to repair a hole between the left and right ventricles, a frequent congenital defect in children, could also find applications in adult cardiac surgery, where similar defects may occur as the result of disease. And so doctors, engineers, and researchers plug away, seeking advances that may someday save the lives of a handful of children, with the hope that some of these discoveries might also find a wider application to other conditions and age groups.
Their work ranges from novel applications of existing technology, in some cases already being applied clinically today, to cutting-edge research exploring fundamental processes and technologies at the very edges of current capabilities. In many cases, such advances may take as long as a decade to bring into practical use, and some are likely to turn out to be too complicated, too expensive, or simply not effective enough. But in the long run, those that do come to fruition might profoundly affect or even save the lives of infants who today face limited options.
From the Inside Out
Dupont, director of the Pediatric Cardiac Bioengineering Lab at Boston Children’s Hospital and Harvard Medical School, is one of the pioneers in this area. The lab has a half-dozen projects underway, including efforts to use microelectromechanical systems (MEMS) engineering technology as a way to develop the tiny precision tools described previously that could be attached to the end of a new kind of catheter, also developed in his lab, allowing more precise position control.
For most surgery to repair heart defects, Dupont says, “you enter the heart through the heart walls. You stop the heart, you cut it open, yet most of these surgeries involve repairs inside the heart.” In the case of some very simple repairs in adults, these can be done through a catheter inserted into the heart via a blood vessel, for example, in the neck. But such surgery is very limited because the tip of the catheter, to be flexible enough to thread its way to the site, ends up not being able to exert much force or to be positioned with much accuracy. That is where Dupont’s lab is trying to make a difference, by creating catheter-based tools with pinpoint precision and the ability to push and pull on tissues to make repairs.
“The risks of surgery are high, so if anything can be done by catheter, that’s the ideal procedure,” he says. “We set out to develop the technology that would enable this. We’re trying to develop fundamental building blocks for surgery that would be able to manipulate tissue. You have to pull and push, cut pieces out, and attach pieces together. And we want to do this inside the beating heart.”
So far, the lab has developed some promising approaches using devices built up through layers of metals by vapor deposition to form gears, linked chains, and other parts, essentially already preassembled when they are removed from their silicon wafer substrate. The MEMS-built tools developed so far include devices that can latch onto tissue at both sides of such an opening and then ratchet the two sides together so they can naturally fuse (below). The kinds of tiny tools the team developed “are clearly what the next generation of devices should look like,” Dupont says.
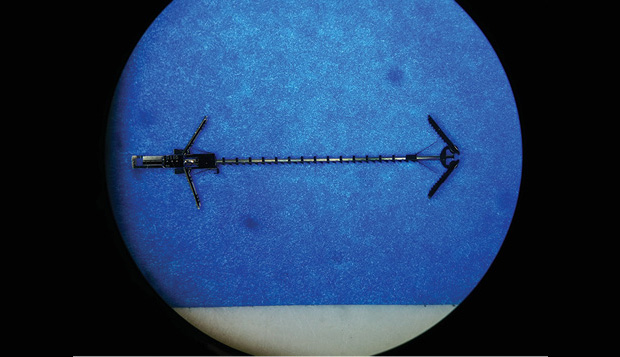
The research will continue with animals for the next two years and, hopefully, get to the point where it is clear whether it is better than existing therapies, he says. In addition, to address the issue of how to achieve precise control over the positioning of the catheter tip, Dupont is working on pressure sensor “skins” that can be applied over the catheter, providing a way of sensing exactly where the device is, how it is moving, and how much resistance it feels from the surrounding tissues. “The goal is to create inexpensive, disposable skins that you can slide over your catheter or your endoscope and measure its position and shape,” he says.
Catching It Early

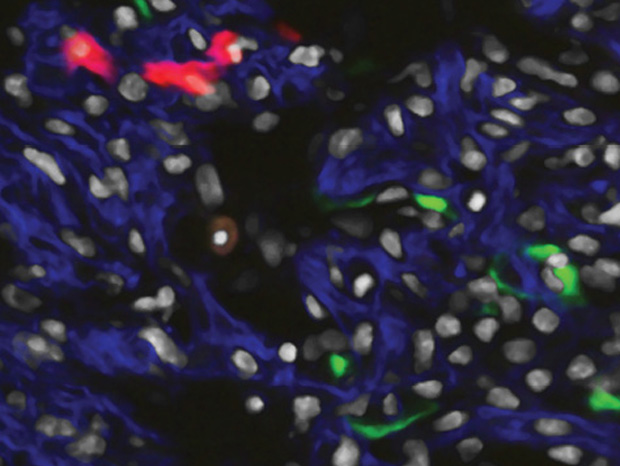
Meanwhile, other labs are tackling the childhood heart problems by trying to catch them as early as possible and finding ways to detect incipient problems before they cause obvious symptoms and while there is more time to plan and implement interventions. For example, at the Gladstone Institute of Cardiovascular Disease at the University of California at San Francisco, Associate Director Benoit Bruneau has been studying the development of the embryo to find out exactly how early specific heart-related structures can be identified (Figure 3). The results have been quite surprising.
“We’ve been studying the origins of the heart, and just how early the precursors get set up,” Bruneau says. In a paper published in January 2015, he and his colleagues showed that cells that would become the left and right ventricles of the heart were already absolutely differentiated from one another much earlier than had been thought (below).
“There’s a supersharp line of demarcation,” he says—and that line constitutes the boundary that will ultimately develop into the intraventricular septum. “It’s defects in the formation of this septum that are the origin of many congenital heart defects,” so being able to identify these structures could potentially lead to earlier diagnostic systems, he says. “If it’s really fundamental, these would be the cellular pathways we would want to target, either therapeutically or in terms of intervention.”
For example, in the case of parents who know they have a genetic susceptibility to heart disease, embryos created through in vitro fertilization could be tested for the genetic marker, and ones found to be free of the disease-promoting gene could be selected for implantation. Or if the defect was found to be present in a developing fetus, plans could be made to be ready for surgical intervention immediately at birth, or even in utero.
He says that in many cases, the evidence so far suggests “the genetic basis for the heart defects may be in the epigenetic regulators, in proteins that will modify the gene or in pathways that turn on or off the gene,” rather than in the genes themselves. By understanding these pathways better, and what regulators affect their progress, it may become possible ultimately to home in on specific regulatory mechanisms that occur across a variety of different specific susceptible gene mutations, and thus may provide especially suitable targets for interventions. “If you understand the heart’s development, you’ll be able to understand what goes wrong in congenital heart defects,” Bruneau says.
Targeting the Genes
New research on precision methods of editing the genome itself is also under development, which could someday make it possible to make specific corrections to the genetic mutations responsible for congenital defects. Though the application of such new techniques clinically may still be a decade off or more, the potential exists for a truly revolutionary approach to the treatment of disease—in some cases, long before it even begins to manifest.
For example, at the Aab Cardiovascular Research Institute at the University of Rochester, Joseph Miano is developing a new technique for precision gene editing, which theoretically should make it possible to correct genetic defects, once their location and action has been identified, to fix the problem at its source.
The method, described in the journal Arteriosclerosis, Thrombosis, and Vascular Biology in February 2015, uses a variant of a precise kind of molecular scissors known as CRISPR. This particular version, called CRISPR-Cas9, has now been demonstrated to be capable of removing and replacing an exact sequence of DNA base pairs from a mouse gene. This represents a dramatic advance over earlier genetic modification methods, where genes were inserted into a target genome essentially in a random location.
The overall CRISPR technology has been virtually exploding since it was first harnessed in 2007, Miano says, with more than 1,000 research papers published on it just last year—more than half of the total published so far. (For more information on CRISPR technology, see Shannon Fischer’s article “Forecast 2014” in the January/February 2014 issue of IEEE Pulse.)
The technique had been expensive and difficult, though amazingly precise and powerful, he says. But new developments have now made it much simpler and less expensive, potentially opening up a wide new area of study. The method makes it possible to select, using customized strands of DNA, an exact location along the 3 billion base-pair strands of DNA where the double strand will be cut. Then, a separate snippet of the desired sequence gets inserted in the gap as the cell’s natural repair mechanisms rejoin the severed strands.
Exactly how these dramatic new capabilities will end up being incorporated into clinical practice remains to be seen, Miano says, but it is possible that once specific genetic variants have been identified that lead to heart defects, people who carry those variants could have the DNA in their sperm or egg cells edited to correct them. Such precise and detailed interventions at a clinical level based on these findings may be far in the future, but other novel applications of new technology are already having an impact on pediatric cardiac surgery today.
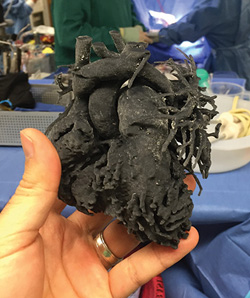
For example, at the Miami Children’s Hospital, Director of Cardiovascular Surgery Redmond Burke has used three-dimensional (3-D) printing technology to allow for more precise planning of pediatric cardiac surgery—something that has so far only been tried in a handful of places (right). Before treating a four-year-old girl with a particularly rare and tricky to repair heart defect called total anomalous pulmonary venous connection, Burke printed a detailed replica of her heart using flexible material.
“I thought that holding and manipulating a flexible 3-D replica of this child’s heart might allow me to plan an operation that hadn’t been done before, configuring the necessary patches to create the exact shapes and dimensions to match her deformed pulmonary veins,” Burke said at a press conference. Dr. Nancy Dobrolet, a pediatric cardiologist at the hospital, says, “3-D printing adds another element in caring for extremely complex conditions where surgical intervention is not typically thought possible.”
The technique has been used successfully in several places so far. Last year, surgeons at Kosair Children’s Hospital in Louisville, Kentucky, printed a 3-D model of the heart of a 14-month-old boy whose heart had multiple defects and used it to plan surgery that was carried out successfully to repair those defects. And last year at Morgan Stanley Hospital in New York, the same process was used to plan surgery for a two-week-old baby with multiple heart defects. “The baby’s heart had holes, which are not uncommon with CHD, but the heart chambers were also in an unusual formation, rather like a maze,” said Dr. Emile Bacha, head of cardiac surgery at Columbia Presbyterian Hospital, who performed the surgery. “In the past, we had to stop the heart and look inside to decide what to do. With this technique, it was like we had a road map to guide us. We were able to repair the baby’s heart with one operation.”
As a method that does not require any special U.S. Food and Drug Administration testing or approval, that is a process that can be implemented right now by any surgeon who chooses to. But it is just one more example of the way people involved in the care of the youngest patients are seeking to apply the latest technologies, from 3-D printing to gene editing to MEMS chip-making, to make a difference for young lives at risk. And that is a future for young cardiac patients that is truly “heartening.”



