Wounds, especially chronic wounds, represent a significant clinical, social, and economic challenge. A recent retrospective analysis of Medicare beneficiaries in the United States identified that about 8.2 million people had at least one type of wound, with surgical wounds and diabetic ulcers among the most common and expensive to treat. The study also found that Medicare expenditures related to wound care are far greater than previously recognized; estimates for acute and chronic wound treatments ranged from US$28.1 billion to $96.8 billion [1].
But even with the annual wound care products market expected to reach $15-–$22 billion by 2024, many aspects of wound care have remained unchanged for decades [2]. Now, teams of clinicians and engineers are working on new technologies that have the potential to transform wound care, making the process smarter, faster, and more efficient.
Innovative and safe treatment
Much of the research of Jeffrey Catchmark, a professor of agricultural and biological engineering at Pennsylvania State University, is focused on developing naturally derived biomaterials to reduce the world’s reliance on plastics. In an attempt to come up with a Styrofoam replacement, he combined an anionic starch with a cationic chitosan (a sugar obtained from the outer skeleton of shellfish) to make a stable, insoluble foam.
Catchmark’s material caught the interest of Scott Armen, chief of the division of trauma, acute care, and critical care surgery at Penn State Health Milton S. Hershey Medical Center and a colonel in the U.S. Army Reserve. He saw its potential in wound care. Together, Armen and Catchmark developed the material into a biofoam that could be placed within traumatic wounds to stop bleeding and stabilize patients [3].
The material starts off as more of a conventional foam, absorbing blood and body fluids and expanding to put pressure on the wound (Figure 1). As healing progresses, it transforms into a gel that aids in wound healing by keeping the area hydrated. In addition, the biofoam has several appealing attributes. Its ingredients are bioabsorbable and safe for long-term use. Chitosan appears to promote clotting to help stop bleeding. It is also flexible and durable, able to be molded to fit inside wounds. “There’s just no other product out there that does all these things in this way,” says Catchmark.

At this time, the biofoam is still in development (Figure 2). Catchmark and Armen are collaborating on a grant from the U.S. Army Medical Research and Development Command to conduct studies of the material in living animals. Their hope is to produce a to-go pack of the foam that could be easily carried and used in the field by emergency responders and military medics.

“This material could ultimately become a common wound care material for almost any application: traumatic wounds, surgical wounds, or everyday wounds,” says Catchmark.
“It could really impact lives, all the way from soldiers on the battlefield to patients in hospitals or even home care.”
On-demand, smart solution
While on a sabbatical at Harvard Medical School, Sameer Sonkusale learned that wound care was a top concern of the doctors and clinicians he met (Figure 3). “We’ve been using the same old bandages to treat wounds and the outcome hasn’t improved,” says Sonkusale, a professor of electrical and computer engineering at Tufts University. That observation inspired Sonkusale, along with collaborators from Harvard and Purdue University, to develop a way to monitor wound healing in real time and deliver treatments on demand.

The result is a prototype for a smart bandage. Demonstrated effective at improving healing in diabetic mice, it can actively monitor different wound biomarkers and deliver appropriate drug doses with little to no intervention needed [4]. Sonkusale says the emergence of flexible electronics made it possible to design a smart material that is also flexible and thin enough to serve as a bandage. The prototype bandages contain heating elements and heat-responsive drug carriers capable of delivering tailored doses of antibiotics or painkillers in response to embedded pH and temperature sensors, which track infection and inflammation (Figure 4). By providing real-time data on how wounds are healing and delivering medicine when necessary, smart bandages like these could save time and money for patients and doctors.
“It’s a problem when wounds become infected and then require more intensive treatment,” says Sonkusale. “What we tried to do with this bandage is create a closed-loop system that monitors the sensors and then delivers the drug on demand.”
Although this particular prototype was equipped with pH and temperature sensors and antibiotic drug delivery, Sonkusale imagines a wide range of potential applications. The type of sensors embedded in the bandage and the treatments delivered could be individualized to monitor different healing markers and treat different conditions.
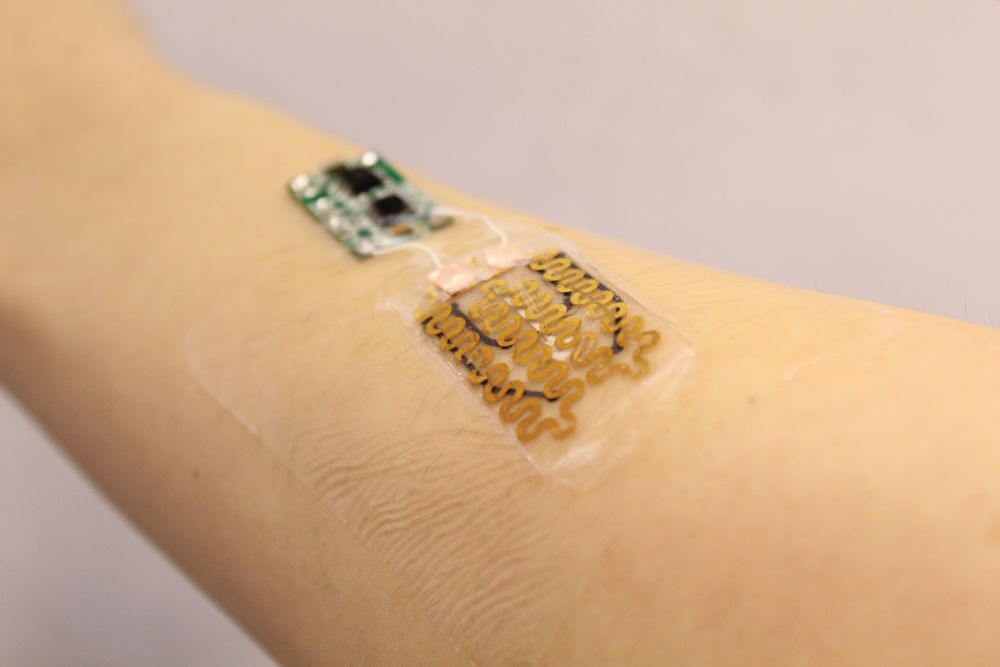
Clinical trials are still needed, but Sonkusale sees a lot of opportunities for improvement to the smart bandage going forward.
“We made versions of the bandages with some features to show we can do this,” he says. “But as a platform, our smart bandage is widely applicable to any kind of wound, including burns, infections, and diabetic wounds.”
Remote, data-driven management
Chronic and nonhealing wounds, such as diabetic ulcers, affect millions of Americans. One recent study showed nearly 15% of Medicare beneficiaries require treatment for at least one type of chronic wound or infection [1]. Responding to this need, a team from the University of Nebraska-Lincoln, Harvard Medical School, and the Massachusetts Institute of Technology designed a smart bandage that can precisely deliver different medications to hard-to-treat wounds [5].
“This bandage combined two advancements,” says Ali Tamayol, one of the device’s inventors who is now at the department of biomedical engineering at the University of Connecticut. “One is mode of drug delivery and the second is in precision of drug delivery.” This smart bandage is equipped with miniature needles that are able to penetrate into deeper layers of the wound, delivering drugs intradermally with minimal pain and inflammation. The needles are controlled wirelessly, allowing treatments to be programmed by care providers from afar.
In a pilot study, diabetic mice treated with the smart bandage showed signs of complete healing and lack of scar formation. The method improved the rate and quality of wound healing compared to topical administration of drugs.
Tamayol says it was important for him and his collaborators to listen to care providers and patients to understand opportunities for improvement. A top concern of patients was minimizing the need for visiting care providers so often. Care providers were interested in measures that could inform them about the conditions of the wound environment. Tamayol believes this smart bandage could do both.
Tamayol says that some of the components of the smart bandage could be altered to fit the needs of different types of wounds. While he is excited by the bandage’s potential, he also acknowledges the challenges of bringing such a product onto the market soon.
“There are a lot of advancements put forward in the engineering world but getting them into clinical practice is not always feasible,” says Tamayol. “The reality might be that simplified versions of some of these technologies can be incorporated into clinical practice initially and then, slowly, more complexity could be added.”
Leveraging natural movement
Xudong Wang, a professor of materials science and engineering at the University of Wisconsin-Madison, has been working on generating energy from the human body for over ten years, developing wearable devices for converting body motions, such as muscle stretching, into electricity. He’s recently turned his attention to the role electrical pulses play in biological processes, with an eye toward medical applications.
“Skin wounds actually use the internal electrical field on the wounded area to help with cell proliferation,” says Wang. “This is how we came up with our hypothesis that we can use this body-generated electricity to stimulate wound healing and facilitate recovery.”
Wang and his colleagues developed a bandage that speeds up healing by harnessing the body’s own energy [6]. The bandage uses body movements to generate gentle electrical pulses at the site of an injury, encouraging wound healing. It consists of nanogenerators that convert movements, such as ones caused by breathing, into electrical pulses (Figure 5). The nanogenerators are linked to a band worn around the patient’s (or lab animal’s) torso. The expansion and contraction of the wearer’s ribcage during breathing powers the nanogenerators, which then deliver low-intensity electric pulses to the wounded area.
In laboratory tests, rats whose wounds were treated with the electric bandage healed in three days, compared to a normal two-week healing process. The results are encouraging, but Wang says further tests are needed before the technology moves into human trials. He is currently collaborating with Angela Gibson, a trauma and burn surgeon at the University of Wisconsin, to test the bandages on pigs, whose skin is more similar to that of humans, and human skin cells in the lab.
“We have tested this on acute wounds, which provide a good example for us to study how the cells are responding, but eventually we want to apply this to more serious and challenging wounds, such as chronic wounds and burns,” says Wang. “Burn wounds can cover a large area and often leave scars. Electric fields are directional and this can help cells align and therefore minimize scar formation.”
Wang believes the technology, which is streamlined and inexpensive, could be put to many different therapeutic uses beyond wound care. He’s also demonstrated the power of his nanogenerators to encourage the growth of hair follicles and stimulate the vagus nerve near the stomach as a way to limit food intake and control obesity.
References
- S. R. Nussbaum et al., “An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds,” Value Health, vol. 21, no. 1, pp. 27–32, Jan. 2018, doi: 10.1016/j.jval.2017.07.007.
- C. K. Sen, “Human wounds and its burden: An updated compendium of estimates,” Adv. Wound Care, vol. 8, no. 2, pp. 39–48, Feb. 2019, doi: 10.1089/wound.2019.0946.
- “Building a better bandage,” Pennsylvania State University. [Online]. Available: https://impact.psu.edu/story/building-a-better-bandage
- P. Mostafalu et al., “Smart bandage for monitoring and treatment of chronic wounds,” Small, vol. 14, no. 33, Aug. 2018, doi: 10.1002/smll.201703509.
- H. Derakhshandeh et al., “A wirelessly controlled smart bandage with 3D-printed miniaturized needle arrays,” Adv. Funct. Mater., vol. 30, no. 13, Mar. 2020, doi: 10.1002/adfm.201905544.
- Y. Long et al., “Effective wound healing enabled by discrete alternative electric fields from wearable nanogenerators,” ACS Nano, vol. 12, no. 12, pp. 12533–12540, Nov. 2018, doi: 10.1021/acsnano.8b07038.



