When it comes to cancer, there’s good news and there’s bad news. While the combination of new screening tests and therapies are making headway, some types of cancer are becoming more common, especially in certain age groups. So how well are we actually doing in the fight against the diseases we collectively call cancer?
The SEER Cancer Statistics Review [1] is a good place to begin answering that question. Issued every year by the National Cancer Institute (NCI), the report tracks rates of incidence (number of new cases reported) and mortality (number of deaths) in the United States. The 2019 report includes incidence data collected by the NCI’s Surveillance, Epidemiology, and End Results (SEER) Program from 1975 to 2016, and mortality data collected by the National Center for Health Statistics. It encompasses information on more than 40 different types of cancer, including the four most common cancers: colon and rectum, lung and bronchus, female breast, and prostate, according to Eric Feuer (Figure 1), Ph.D., chief of the NCI’s Statistical Research and Applications Branch and a contributor to the report.
“If you look at all cancer types combined, you can see that cancer mortality and incidence overall are going down, so that’s the broadest picture,” Feuer said. “But then to think about why that’s happening, it can be pretty complex. Each cancer has its own story.”
Tricky analysis
Prostate cancer illustrates how data are important, but don’t always paint the full picture. Feuer pointed to a near doubling in the incidence of prostate cancer, jumping from 138/100,000 individuals in 1988 to nearly 240/100,000 in 1992 (Figure 2). “That huge spike is one of the most dramatic things that’s ever happened for any cancer, but the reason behind it is that prostate-specific antigen (PSA) screening was introduced in the population very rapidly at that time, so the early detection was essentially pulling cases from the future to the present,” he explained. Once PSA became a common screening tool, incidence quickly leveled off, dropping to 160/100,000 three years later. It has been declining from 2000 through 2014, and now stands at about 110/100,000, similar to the rate in the mid to late 1970s.

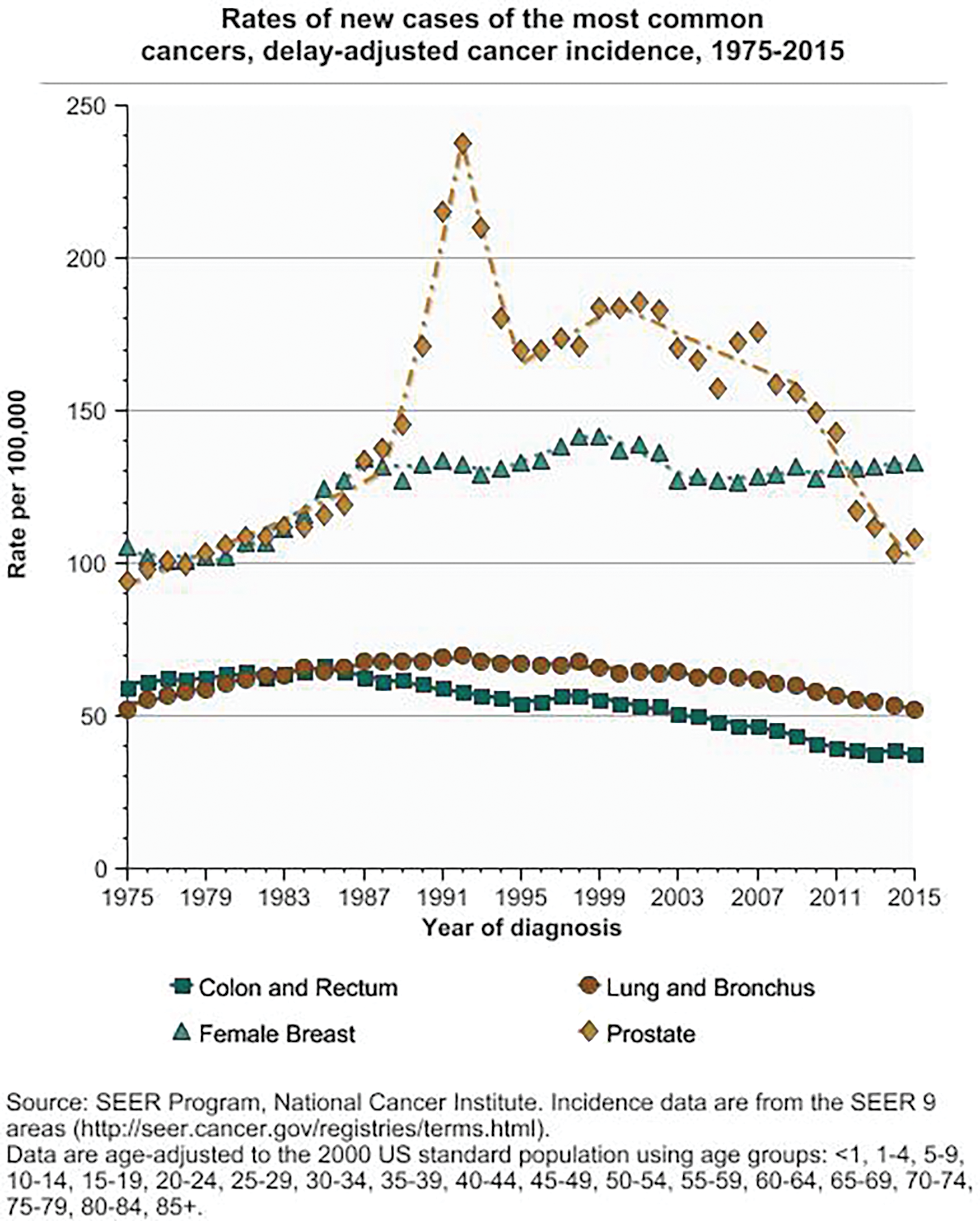
The increase in incidence could, therefore, be seen as a good thing because it was picking up cancer early on and usually well before any symptoms appeared. “Yes, detecting asymptomatic cancer has many upsides, but it also has potential downsides,” Feuer said. “One of the downsides is false positives and potential complications from biopsies, but the bigger downside is that we can overdiagnose.” He explained that while tests may be able to find asymptomatic cancers, they can also lead to treatment for cancers that might not actually become symptomatic during a patient’s lifetime. “And if it wouldn’t become symptomatic, then what we’re doing is treating patients somewhat unnecessarily,” he said.
This potential for overdiagnosis has been an issue with the PSA test for prostate cancer, he remarked. “In two major randomized, controlled trials of PSA screening done in different settings and under different protocols [2], one showed that it reduces mortality, but the other one didn’t. And even if it does reduce mortality, there’s a tradeoff with overdiagnosis of disease,” he said. However, prostate cancer mortality has been falling steadily from 1993 through 2013 from a high of about 39.3/100,000 to 19.3/100,000 likely due to both screening and advances in treatment.
The story of female breast cancer trends (Figure 3) is even more complicated, Feuer said. Incidence started increasing in the early 1980s when mammography screening was introduced, but the screening is likely not the only major driver of incidence rates. “For breast cancer, there are many risk factors and protective factors, such as early age at first menses, hormone replacement therapy, obesity, starting menopause at an older age, never having a full-term pregnancy, and having a first pregnancy after age 30. With so many risk factors that can be moving in different directions, it’s a pretty complex picture,” he said.
In addition, mammography can find abnormal cells inside a milk duct (called ductal carcinoma in situ or DCIS), but some in situ cancer has a low risk of becoming invasive. “And in situ was not commonly detected prior to the introduction of mammography, so some portion could be over-diagnosed,” he said. “Since it is not always clear which in situ cancers will progress, it is typically treated, although there are several trials under way evaluating active surveillance vs. standard therapy for DCIS that is considered to be at low risk of progressing” [3]–[5].
On the mortality side, breast cancer has experienced a “wonderful decline” for several reasons, Feuer noted. According to the 2019 Cancer Statistics Review, overall mortality in the U.S. has steadily dropped from about 31.6/100,000 in 1992 to about 20.0/100,000 in 2016. “In the early years, the mortality decline was due to a mix of adjuvant therapy after surgery (i.e., multiple-agent chemotherapy and hormonal therapy) and the introduction of plain-film mammography followed by digital mammography, which gradually replaced plain-film mammography between 2000 and 2010,” he said [6]. More recent declines in mortality can be attributed primarily to treatment advances, including new and targeted approaches, such as Herceptin (trastuzumab) for HER2-positive breast cancer.
Colorectal and obesity-related cancers
For colorectal cancer, mortality has fallen by more than 40% from 23.6/100,000 in 1992 to 13.7/100,000 in 2016, and Feuer gives much of the credit to both improvements in treatment, and the increased use of screening methods, especially colonoscopy, which not only finds potentially precancerous polyps but removes them and therefore prevents cancer. That, however, is not the entire story. An emerging trend in colorectal cancer is its rise in incidence among younger people. And it isn’t the only type of cancer showing this shift.
Researchers at the American Cancer Society (ACS) and NCI pored through data collected from 25 state cancer registries from 1995 to 2014 to understand how cancer affects different age groups, and found that younger generations are facing a heightened risk of diagnosis with certain cancers, especially those that are associated with obesity [7]. Published in March 2019, the ACS study broke down the data into five-year age groups from 25–29 up to 80–84, and assessed each age group over the years (e.g., they contrasted the 25–29 age group in people who were born at different points in time from 1910 through 1989). According to the data, six obesity-related cancers—multiple myeloma, colorectal, uterine corpus, gall bladder, kidney, and pancreatic—have experienced significant rises in incidence among people aged 25–49 years with progressively steeper jumps in successively younger generations, according to Hyuna Sung, Ph.D., lead author of the study and principal scientist in the Surveillance and Health Services Research Program at the ACS.
“Looking at colorectal cancer, for instance, it has increased exclusively among younger adults, in contrast to the decreasing trend among older adults,” Sung said. For pancreatic cancer, although incidence has been ticking upward across all ages, a more rapid increase occurred among younger than among older adults: Between 1995 and 2014, pancreatic cancer rose 4.3% per year among those aged 25–29, compared to less than 1% per year in those 40 years or older, she noted. “And it’s not just happening in the U.S. A similar trend, especially for colorectal cancer, has been reported in several other high-income countries.”
Additional data showcases the increase in overweight and obesity, which refer to a body-mass index of at least 25 or 30, respectively. Worldwide, the proportion of adults aged 20 and older who are overweight or obese surged from 21% in 1975 to 39.9% in 2016 for men, and from 24% to 40.5% for women. Among those aged 5–19 years, the percentage who were overweight or obese more than quadrupled from 1975 to 2016, rising from 5% to 27% for boys, and from 6% to 24% for girls [8]. Some analyses predict that 58% of the world’s adult population will be overweight or obese by 2030, and in the U.S., that number could top 85% [9].
The trend is particularly troubling for the future, Sung said. “The effect of the obesity epidemic on overall cancer burden has not yet fully manifested. These young adults are carrying their body weight as a carcinogenic factor over the long run—for their lifetime—so the cumulative exposure to excessive body weight will be longer among these young people compared to their counterparts in older generations,” she reported.

“Our study demonstrates the epidemiological transition in cancer risks: While smoking- or infection-related cancers were more serious concerns for the previous generations, younger generations are experiencing higher risks from obesity.”
Tech trends
Technology has and will continue to shape cancer trends in a number of ways, according to cancer genomics expert and researcher Elaine Mardis (Figure 4), Ph.D., who is currently employing knowledge gained from next-generation sequencing platforms to explore pediatric cancer diagnosis and treatment decision-making. She is also president of the American Association for Cancer Research, co-executive director of the Institute for Genomic Medicine at Nationwide Children’s Hospital in Columbus, OH, and a co-leader of the Translational Therapeutics Program at The Ohio State University Comprehensive Cancer Center—James.
Imaging technology is in an especially exciting transition. “The advances in imaging technology are becoming ever-more sensitive and specific, both of which are important for picking up earlier cancer lesions and for less invasive disease monitoring,” Mardis said. Fluorodeoxyglucose-positron emission tomography (FDG-PET) has been an excellent tool for imaging the metabolic activity rate of cancer, she remarked, and its benefits could be enhanced by new work exploring the addition of high-resolution nuclear magnetic resolution (NMR) spectroscopy to evaluate cancer-specific metabolites. She foresees research aimed at miniaturization of handheld imaging devices as a future step forward.
“Also from my angle in genomics, we have now very complementary technologies that are helping with early detection, including what’s commonly referred to as liquid biopsy, so using blood, urine, stool and other analytes to very sensitively detect cancer mutations,” Mardis said, noting that liquid biopsies can hone in on identifying traces of cancer cells’ DNA. “I’m anticipating in the future that during your annual checkup at the doctor and while you’re giving blood for (typical) tests, such as cholesterol level, you’ll give another vial of blood for cancer screening.” Since the most common cancers in humans are characterized by a small number of mutations, she added, she envisions “a fairly inexpensive, rapid test that could even be done in the doctor’s office” to tell the patient whether any signs of potential cancer are present and require follow-up.
Mardis singled out sequencing technology as another area of advancement that has had a profound impact on the understanding of cancer and is now a part of medical care of cancer patients. This area includes work begun with the Human Genome Project following its sequencing in 2003, expanded through such projects as The Cancer Genome Atlas that has detailed more than 30 cancer types so far [10], and additional research, such as the Pediatric Cancer Genome Project [11] that characterized more than 800 pediatric cancer genomes (Figure 5), she said. (Mardis was a co-leader of the Pediatric Cancer Genome Project.) The advent of single-cell sequencing is now also making possible detailed studies of the heterogeneous mixture of cell types, including rare cells, involved in the progression of cancer, she stated.

The rapidly evolving field of wearable technologies will also become important through its ability to continuously collect physiological and other data about individuals (the so-called digital exposome), she said, reporting these data automatically into personal databases or electronic medical records.
“In all of this technological progress, computational analysis and database technology has had and will have a profound impact,” Mardis asserted. “We continue to build large databases that allow each individual patient to benefit from the legacy of other patients in the context of more accurate diagnosis and more accurate treatment. Hopefully, full use of database information will continue to help patients future-forward as we develop new therapies.”
Prevention is one area that could use more of the tech spotlight, especially in light of the ACS study on the increased incidence of obesity-related cancers among younger adults, according to Sung. “Most biomedical technologies are heavily focused on development of new treatment, such as immunotherapy and targeted drugs, which are both very important, but as we gain more knowledge about cancer risk factors, and especially modifiable risk factors, I would like to see cancer-related technologies that can innovate cancer-prevention strategies,” she said. “Given the prevalent evidence, here in the United States in particular, too many kids are predicted to carry a long, cumulative exposure of excessive body weight, so I hope our study calls more attention to the need for targeted prevention efforts for childhood and early-adulthood obesity, because early life exposures matter when it comes to cancer risk.”
So how are we doing in the fight against cancer? “It remains a vexing problem,” Mardis said, but quickly added that screening tests, new therapies, and a better understanding of cancer are together landing some solid blows. She commented, “I just feel like the field in many ways is exploding with the data and knowledge that we have now. It’s really remarkable. But at the same time, there’s much more that needs to be done.”
References
- N. Howlader et al. (Eds.), SEER Cancer Statistics Review, 1975–2016. Bethesda, MD: National Cancer Institute, Apr. 2019. [Online]. Available: https://seer.cancer.gov/csr/1975_2016/
- K. Rove and E. D. Crawford, “Randomized controlled screening trials for prostate cancer using prostate-specific antigen: A tale of contrasts,” World J. Urol., vol. 30, no. 2, pp. 137–142, Apr. 2012.
- A. Francis et al., “Addressing overtreatment of screen detected DCIS; the LORIS trial,” Euro. J. Cancer, vol. 51, no. 16, pp. 2296–2303, Nov. 2015.
- L. E. Elshof et al., “Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ—The LORD study,” Euro. J. Cancer, vol. 51, no. 12, pp. 1497–1510, Aug. 2015.
- L. M. Youngwirth, J. C. Boughey, and E. S. Hwang, “Surgery versus monitoring and endocrine therapy for low-risk DCIS: The COMET trial,” Bull. Amer. College Surgeons, vol. 102, no. 1, pp. 62–63, Jan. 2017.
- S. K. Plevritis et al., “Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000–2012,” JAMA, vol. 319, no. 2, pp. 154–164, Jan. 2018.
- H. Sung, R. Siegel, P. S. Rosenberg, and A. Jemal, “Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry,” Lancet Public Health, vol. 4, no. 3, pp. e137–e147, Mar. 2019.
- NCD Risk Factor Collaboration, “Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults,” Lancet, vol. 390, pp. 2627–2642, Dec. 2017.
- A. Hruby and F. B. Hu, “The epidemiology of obesity: A big picture,” Pharmacoeconomics, vol. 33, no. 7, pp. 673–689, Jul. 2015.
- “The Cancer Genome Atlas Program,” National Cancer Institute. Accessed: Dec. 2, 2019. [Online]. Available: https://www.cancer.gov/ about-nci/organization/ccg/research/structural-genomics/tcga
- “Pediatric cancer genome project,” St. Jude Children’s Research Hospital, Memphis, TN, USA. Accessed: Dec. 11, 2019. [Online]. Available: https://www.stjude.org/research/pediatric-cancer-genome-project.html



