The idea is a compelling one: a device that looks and feels like an ordinary contact lens but that can continuously monitor a variety of health indicators. For a diabetic, such a lens might update blood glucose levels and, using a built-in flashing LED indicator light, signal when a condition needs attention. Diabetic patients might be saved from the need for repeated finger prick tests and could be monitored for longer periods of time and for a greater variety of parameters at once.
It’s a vision that over the last several years has drawn serious interest and research efforts from the likes of Google, Apple, Novartis, Sony, and Samsung (or their subsidiaries or spinoffs), all of which are said to have ongoing projects to develop such devices. The companies have mostly been secretive since their initiatives were launched about four years ago, and the field is already littered with a long history of bold promises made for diagnostic contact lenses that were said to be imminent but whose promise remains unfulfilled.
A Success with Triggerfish
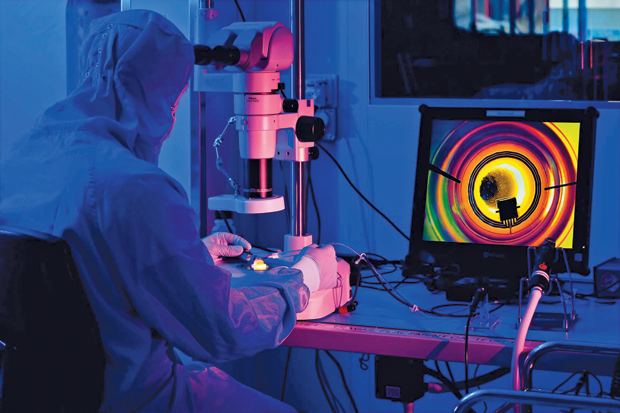
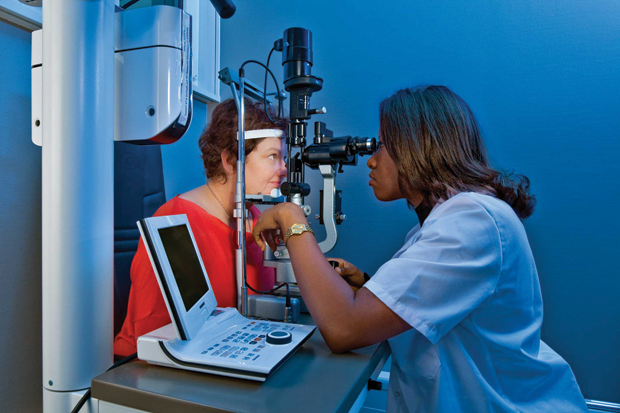
But somewhere, progress is being made. Academic publications from researchers in South Korea and in Japan suggest that a few companies remain clearly committed to the quest. The furthest along seems to be the Swiss company Sensimed, based in Lausanne, which has a patented contact lens system for continuously monitoring pressure in the eye as a way of improving diagnostics for glaucoma patients (Figure 1). The system has undergone years of trials and earned U.S. Food and Drug Administration (FDA) approval last year—the first-ever contact lens-based diagnostic device to do so—thus creating a new FDA category.

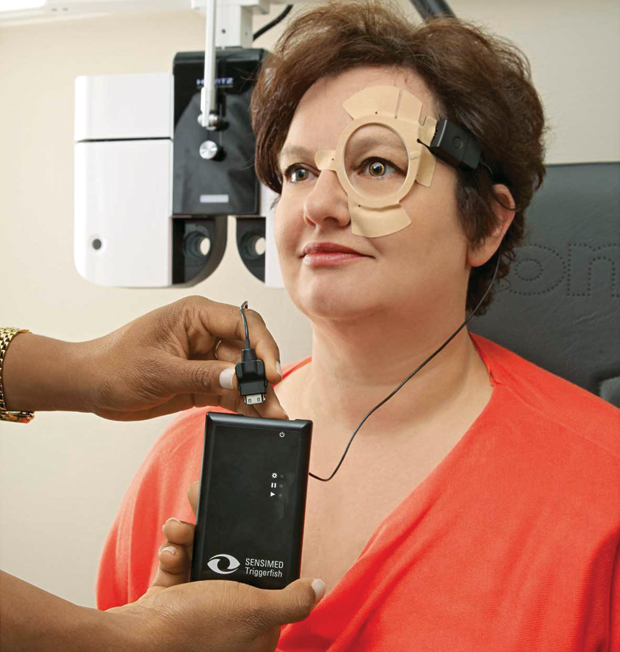
Called Triggerfish (Figure 2), the device is still undergoing testing, says Sensimed CEO David Bailey. It allows a doctor to monitor a patient’s intraocular pressure (IOP) continuously for 24 hours to determine the specific diurnal pattern of pressure variations for that patient. That’s important because for long-term monitoring the usual protocol is to take daily tests; however, pressure levels can vary greatly over time. Clinical studies using the device have shown that it can improve results by allowing clinicians to choose the best times of day for testing or provide a way to correct readings for the time of the measurement.
The device consists of thin, partially transparent electrodes and tiny microchips embedded within the soft plastic material of a conventional contact lens (Figures 3 and 4). It does not directly measure the IOP, Bailey explains. Rather, “What we’re measuring is something similar, which is volume change.” Converting the changes in volume to a measurement of IOP requires some adjustments for other factors that can affect the volume, including temperature. The company now has a database of results from more than 2,000 patients, mostly with glaucoma but also some control subjects without it, allowing researchers there to home in on the most accurate conversion factors.
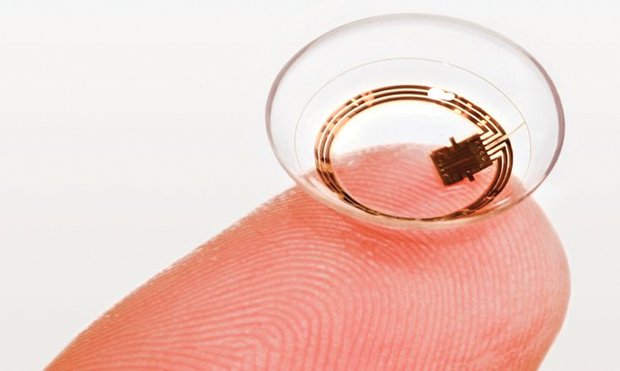
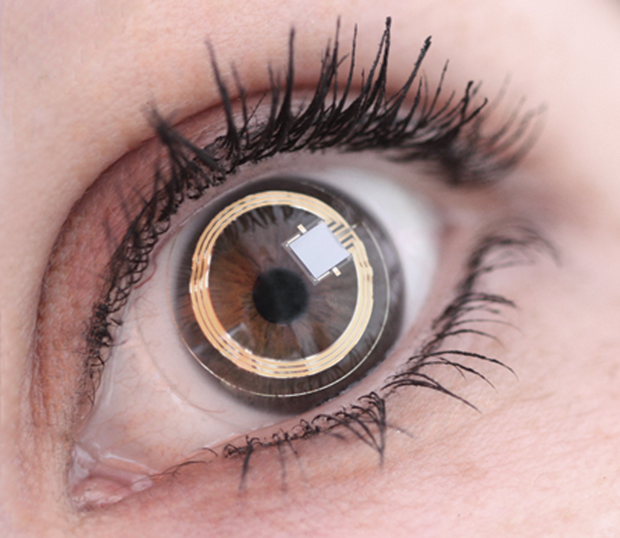
Pressure is not the only factor affecting the progression of glaucoma, Bailey says. There is a variant of the disease, especially prevalent in Asia, that is not correlated with pressure at all. Using a system like Triggerfish would allow doctors to screen out those patients for whom conventional pressuremonitoring regimes might not be useful (Figures 5 and 6).
Triggerfish has been in use by researchers in several countries in Europe for years and will likely be made available by early 2019 in the United States and Japan, where regulatory approval is expected imminently. Once approved, the product will be sold to practitioners for use in their diagnostic process, Bailey says. He doesn’t foresee any patient- or consumer-level version for such a product but says the company does intend to incorporate other possible diagnostic measures beyond the IOP. “We’re pursuing other sensors to go into contact lenses.”
Great Expectations
But, in the meantime, quite a few other companies and research teams are slowly plugging away at the overall goal of noninvasively measuring key health indicators, such as blood glucose. Using tears rather than blood has long seemed like a promising alternative, offering a device that could comfortably remain in the eye while providing continuous measurements and, in some versions, even include a built-in warning system, such as a light or a color change, to indicate a need for intervention.
For example, a South Korean team led by Jang-Ung Park, a professor of materials science and engineering at Ulsan National Institute of Science and Technology, reported early this year on preliminary tests of a soft contact lens containing a glucose sensor and an LED light. The lens could provide a visible color indicator when readings suggest incipient hyper- or hypoglycemia and thereby provide early enough warning to take action and prevent adverse reactions, which can include fainting and even death. The device has been tested only in rabbits so far, with no adverse reactions, as stated in the team’s report. Work has been continuing, and the group hopes to be ready for human trials soon.
Such a device would require stringent testing for FDA approval, which would likely take several years, and some researchers say a number of hurdles remain to overcome. The detection system used by the Park team relies on a chemical reaction between glucose and a reactant preloaded into each lens. That chemical declines over time, and the results need to be adjusted for the decline. Also, as David Walt of Harvard Medical School and the Wyss Institute has pointed out, the reaction produces hydrogen peroxide as a byproduct, which could potentially be damaging to the eye.
But, overall, Walt thinks the challenges are surmountable. He notes that, as with tears, “there are areas of the body (e.g., skin) where the glucose levels lag behind the levels in blood, but that is not a major concern if one understands the lag time. In such cases, one can use the rate of change to predict what the level will be, rather than the instantaneous measurement.” Moreover, “For most applications where continuous measurements are made,” Walt continues, “the goal is to look at the trend and predict when one needs to either administer insulin, for hyperglycemia, or ingest sugar, for hypoglycemia, to avoid adverse effects. I’m not exactly sure where tears lie on the spectrum of lag times, but a continuous measurement that predicts such changes would be of significant value when validated.”
Glucose-measuring contact lenses have been a holy grail among efforts to find noninvasive alternatives for traditional monitoring devices, such as the pinprick blood tests many diabetics endure often multiple times a day. In January 2014, Google announced with great fanfare just such a project, calling it the inspiration behind the creation of an entirely new spinoff, Verily Life Sciences based in San Francisco. The company has since partnered with Alcon, a subsidiary of Novartis, to develop the technology. The news release and the ensuing stories made it sound just around the corner, but, although the company’s website continues to tout its glucose-monitoring contact lenses as a flagship project, there have been no further announcements or publications about it.
“We do not have any updates to provide on our contact lens program with Alcon at this time,” according to Verily spokesperson Carolyn Wang. However, in addition to two other adjustable vision-correcting contact lens projects, the glucose-monitoring lens remains a current company focus. “These smart lens projects involve bench testing as well as prototype design and creation. We’re learning a lot from each project, are pleased with the progress of the collaboration to date, and are moving at a pace expected in the feasibility stage, or early phase, of development,” Wang explains.
Others, including Sony, Apple, and Samsung, have also been widely reported to be working on similar lens-based diagnostics but have not revealed any details. “There have been lots of promises,” says Bailey, “but I’ve seen nothing delivered at all.”
A Long Road Ahead

Sandy Asher, distinguished professor of chemistry at the University of Pittsburgh (Figure 7), thinks the field has great promise but foresees a long road ahead. Asher was one of the pioneers of the idea of monitoring glucose using tears rather than blood. He began publishing research on such measurements more than a decade ago, using “a piece of a hydrogel and a photonic crystal in a contact lens,” he notes. The device changed colors depending on the glucose concentration so that a patient could look in to a mirror to see the results. Oddly, Asher says, it’s not clear why such metabolites as glucose should appear in tears in the first place. “You could argue that it shouldn’t be there,” he says. “It’s good food for bad bugs.”
But Asher continues to be very optimistic that such diagnostic systems built into contact lenses remain a highly promising field. “There are a lot of things you can potentially do,” he continues. “Tear fluid contains a lot of chemical species, it’s very complex. There may be a number of analytes, probably other carbohydrates, whose levels probably depend on state of health that would be useful to measure. But we don’t yet have any additional targets that we’ve identified.”
Even if glucose were the only useful target for contact lens monitoring, the potential for such a system—if it is proven safe, effective, and affordable—could be enormous. More than 400 million people worldwide are estimated to have diabetes, and its medications and treatments represent a US$10 billion annual market—and a tremendous amount of suffering. “It could be a huge improvement for people who are having terrible complications,” Asher says, “and maybe life-saving for people with hypoglycemia. You’d be able to head off a hypoglycemic event that could cause them to black out and die.”
Having tried and failed to launch a business to develop such devices eight years ago, Asher doesn’t underestimate the challenges that still lie ahead, however. “If it were easy, ”he concludes,“ it would already have been done.”