Ask any surgical oncologist, and you’ll hear the same thing: tumors are insidious. Removing them completely can be very difficult. Sometimes tumors are in hard-to-reach areas, and, in many cases, tumor tissue looks so much like normal tissue that surgeons cannot tell exactly what to excise and what to leave alone.
With a wide range of new technologies becoming available, however, clinicians are beginning to envision a time when they can confidently remove or eradicate tumors precisely and quickly, even in the brain and upper abdomen, where surgeries have been extremely challenging. And they can do so while also greatly reducing patient-recovery time.
Such new technologies include innovative capabilities for distinguishing normal cells from tumor cells, advanced surgical robotics, and emerging radiation technologies designed especially for removing growths that are otherwise hard to reach. For front-line clinicians, these new technologies can’t come soon enough.
Tumor or Normal?

One clinician who is more than ready to turn to new technologies that will take some of the guesswork out of cancer surgeries is neurosurgeon Daniel Orringer, M.D., assistant professor in the Department of Neurosurgery at the University of Michigan and director of the department’s Translational Molecular Imaging Laboratory (Figure 1, right). “When I was a medical student and saw my first brain-tumor operation, I recognized that our ways of dealing with these tumors were actually pretty crude,” he recalls. After removing any obvious tumor, the surgeon had to then play what was effectively a guessing game: Does this bit of tissue look like it might be cancerous? Does that edge feel like it’s normal tissue or not?
Ever since watching that operation 15 years ago, Orringer has been intent on bringing precision to brain-tumor surgery, and in the past year he has been conducting brain-tumor resections using a tool that allows him to discern tumor cells from normal cells within a matter of minutes right in the operating room. “When I sit with my patients before an operation on a brain tumor, I tell them about this constant balancing act, where we want to take out as much tumor as possible because we know that helps their survival, but it cannot come at the cost of taking the adjacent normal brain, because if their brain function is compromised, that actually also has a negative impact on survival,” he says. The balancing act is further complicated by the fact that tumor tissue often looks so much like normal brain tissue that even the most experienced surgeon cannot tell them apart and, therefore, may leave tumor tissue behind.
Over the last year or so, Orringer has found something that works: a new microscope based on technology developed by Prof. Xiaoliang Sunney Xie’s research group at Harvard University [1] and now broadened for clinical use [2]. The microscope provides a quick and easy determination of whether tumor cells are present. As of late September 2016, Orringer and his surgical team had imaged more than 270 patient samples with this microscope (Figure 2).
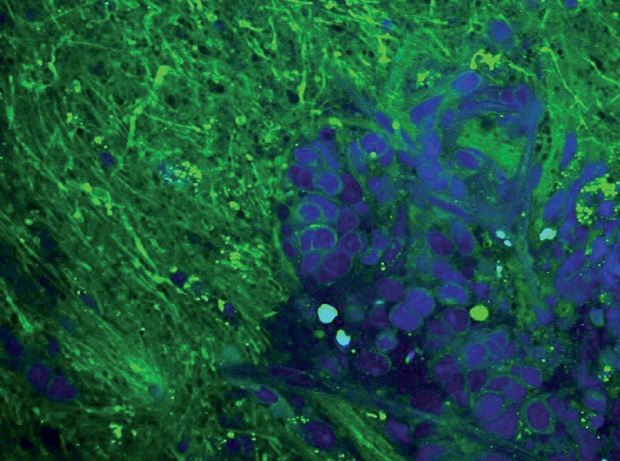
To appreciate the microscope’s capabilities, Orringer first describes the traditional approach. Using computed tomography (CT) and magnetic resonance imaging (MRI) images to initially map the gross tumor, the surgeon takes out the obvious growth. Once that task is completed, the subjective part begins. The surgeon examines the tissue visually or by touch to try to discern tumor from normal tissue. Surgeons can also take a tissue sample and send it to a dedicated pathology lab for analysis. “There, the pathologist freezes it, slices it, stains it, puts it on a slide, and evaluates it,” Orringer explains, noting that the surgeon may have to wait 20–40 minutes for the results. If tumor cells are present, the surgeon resects more tissue and may repeat the process. This back-and-forth to the pathology lab may work, but it is often not practical during brain-tumor surgery, Orringer contends. “During brain-tumor surgery, the surgeon must focus exclusively on taking care of the patient.”
This need for speed is where the new microscope shines. The technology uses laser-based coherent Raman scattering microscopy, which relies on Raman signals amplified 10,000-fold over spontaneous Raman signals through a process called stimulated emission. This vibrates chemical bonds in the sample’s molecules. A computer analyzes those vibrations, which are different in tumor cells versus normal cells, and produces three-dimensional (3-D), highresolution microscopic images that clearly show which cells are tumor cells and which are not. According to Orringer, “What this technology—coherent Raman scattering microscopy—allows us to do is obtain microscopic images of tissues without having to do any preparation to the tissue, because all of the contrast in its images is generated by the inherent chemical makeup of that tissue. And that means we can do it right in the operating room and get images within a few minutes” [3], [4].
The microscope is able to image tissues inside the patient, but Orringer usually uses the microscope on excised tissue samples, which he has found to yield higher image quality. “Either way,” he says, “the fact that the technology provides 3-D optical sectioning, is label-free, and is very fast allows us to obtain the data we need to guide surgery.”
Orringer is an advisor to Invenio Imaging, Inc., of Santa Clara, California; the company is currently commercializing the technology and hopes to begin selling it in 2017. (Both Orringer and Xie hold equity in the company.) Orringer’s research group has also been instrumental in integrating the microscope’s digital images into the electronic medical record. He explains, “The images now come into our scope and can be uploaded into the cloud for viewing from any workstation in the hospital network.”
Orringer is currently working with other clinicians to expand the microscope’s use to additional cancers. “There are many situations in surgical oncology where differentiating tumor from the adjacent tissue is a challenge,” he says. “Breast [cancers], gastrointestinal cancers, and melanoma are all things that can be very difficult to detect visually, and we expect the technology will have an impact on those disciplines as well.”
Better Robots in the Operating Room
Another technology greatly improving cancer treatment is advanced robotics, according to Dwight Im, M.D., an internationally known robotic-surgery expert, leading gynecologic oncologist, and head of the National Institute of Robotic Surgery at Mercy Medical Center in Baltimore, Maryland (Figure 3).
“In gynecologic oncology, robotics has undergone huge advances over the past five to seven years in terms of being able to perform some of the most complex surgeries robotically,” he says. The current star in his operating room is the new Intuitive da Vinci Xi surgical system, which provides the surgeon with super- sharp, high-definition, 3-D images of the surgical field. “The resolution is superior,” Im marvels. “Sometimes I actually have to stop in the middle of the operation, and do you know why? I’m just mesmerized, because I can’t believe what I’m seeing. It’s so beautiful.”
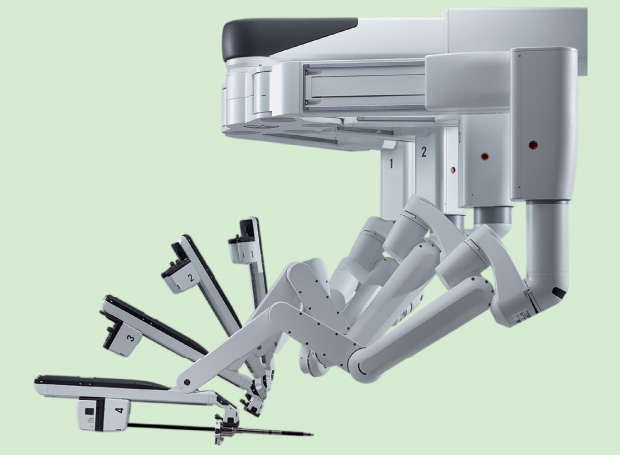
Im is also enamored with the maneuverability and thinness of the Xi’s overhead instrument arms, which are equipped with mechanical wrists that move like the surgeon’s own hand (Figure 4). These allow the surgeon to move around easily and intuitively, even in the hard-to-access upper abdomen, all without having to reposition the patient (Figure 5). In Im’s words, “With the older Si model, we would have to undock the robot, turn the patient nearly 180°, and then redock. And even then, it was not that feasible for most surgeons to do those surgeries. But with the Xi, you can turn the boom around 180° without having to redock. It makes a huge difference and allows us to perform optimal tumor debulking.”
Im conducted his first surgery with the new system in February 2016, and he believes the Xi may be especially useful for removing ovarian tumors after chemotherapy, a somewhat controversial approach that he champions. “The old school view was always surgery followed by chemotherapy, but about five years ago, people started to ask if it would be a better option in some cases to give chemotherapy first to shrink the tumor and then do surgery,” he explains. “Anecdotally, I have seen in some patients who were medically unfit to go through a radical surgery up front—or who made the choice to try it—that they did well when they got three cycles of chemotherapy followed by surgery and then three additional cycles of chemo, compared to radical surgery followed by six rounds of chemotherapy.” In fact, he adds, the chemo-before-surgery option in combination with the improved imaging and surgical maneuverability provided by the Xi can greatly improve the likelihood of complete resection.
This chemo-preceded surgery is also less taxing on the patient, Im notes. The traditional surgery-first approach typically involves a 10- to 12-inch incision and is followed by a couple of weeks in the hospital, including three or four nights in the intensive care unit, and then at least a one-month wait before chemotherapy can begin, he says. In contrast, the chemo-beforesurgery approach can shrink the tumor to about one centimeter, which makes surgery simpler.
This is especially true with the new single-site capability of the Xi system, which allows the surgeon to operate through a tiny incision, according to Im. He performed the world’s first gynecologic single-site surgery with the Xi in March 2016. With chemo first followed by single-site Xi surgery, the patient can go home from the hospital in just one day, resume his or her normal life almost immediately, and begin chemo in as little as two weeks, he contends. “To me, this is a no-brainer. It’s just a better option in many instances.”
Radiation Tech Is Hot

Emerging radiation technologies will also influence the war on cancer, especially when it comes to those tumors that are currently hard to reach, according to Bruce Minsky, M.D., immediate past chair of the American Society for Radiation Oncology and professor of radiation oncology at the M.D. Anderson Cancer Center in Houston (Figure 6, right).
One technology that is gaining prominence is stereotactic body radiation therapy (SBRT), which is used in lieu of surgery. SBRT employs a very high dose of radiation over a small field, usually one to two centimeters, to eradicate the tumor. “We use this technique throughout the body, including the brain, lung, abdomen, and other areas, to treat a single small focus of cancer that might not be surgically accessible,” Minsky explains. SBRT is used in conjunction with imaging, usually CT scans or MRI, to help precisely focus the beam from multiple angles.
SBRT has been available for roughly a decade, but it is only recently that clinicians have begun to fully take advantage of it, Minsky asserts. This is because patients are receiving systematic chemotherapies that have improved their outcomes, and that, in turn, presents the time for the potential development of small residual sites of metastasis that are well-suited for treatment with SBRT, particularly SBRT associated with another new development, MR-guided radiation therapy.
Minsky describes two MR-guided therapy machines, one that is already commercially available and a second that is in development. “These combine the MR and radiation-therapy machine into one, and they are designed to track the tumor as it moves,” he continues, explaining that depending on their location in the body, tumors can move up to an inch as a person breathes in and out. “With this technology, the radiation beam follows the tumor, similar to the way the military can track missiles. In the near future, we can use functional MRI to analyze the metabolism of the tumor as it is being treated so we can see if we need to increase the dosage as well.”
The commercially available MR-guided therapy machine, developed by Accuray, Inc., of Sunnyvale, California, currently has three cobalt sources for the radiation field, while the in-development machine, developed by Elekta, of Stockholm, Sweden, has a linear accelerator as the radiation source, Minsky says. The M.D. Anderson Cancer Center is one member of an international consortium that is developing the latter, which he estimates will be available commercially in the next two years. He adds, “Both of the machines are built to do the same thing: use the MR component to help guide the radiation in real time.”
Beyond these technologies, Minsky is also excited about the next generation of scanning-beam proton therapy, which permits the placement of the highest peak of the radiation at the exact location and depth of the tumor. “We have already started to install these machines, and we have randomized studies ongoing to compare intensity-modulated radiation therapy with photons to intensity-modulated proton therapy (IMPT), which uses scanning beam protons,” he explains. “IMPT uses an elaborate network of magnets to focus the proton beam very precisely and then delivers the radiation to the tumor one extremely thin layer at a time, so it can carve away the tumor bit by bit.”
The hope is that all three of these emerging radiation technologies will improve cure rates because clinicians can pinpoint the tumor. This precision allows them to use higher and more lethal doses of radiation to target the tumor while leaving even closely neighboring normal tissue unscathed, Minsky adds. “With advances like these, I see the role of radiation continuing to increase as a noninvasive technique.”
What’s Next?
The goal of all these technologies is the same: to aim at cancerous tumors, especially those that have always evaded treatment. The field is constantly changing as new technologies undergo development and make their way into hospitals. “There are a lot of things coming,” remarks Orringer. One he finds especially interesting is the promise of fluorescent dyes, particularly a dye called 5-aminolevulinic acid (5-ALA). “What 5-ALA does is cross the blood–brain barrier into brain-tumor cells, and by exploiting metabolic differences in the cells, the compound is metabolized to a fluorescent byproduct,” he explains. “When you excite the tissue at a specific wavelength using some optic filters that can be easily put onto an operating microscope, the tumor will glow orange.” 5-ALA has received approval in Europe to guide brain-tumor surgery, and Constantinos Hadjipanayis at Mount Sinai Medical Center in New York is spearheading the effort to gain similar approval in the United States.
Fluorescent dyes are also in the pipeline for guiding radiation therapy, Im says. He mentions work underway to develop dyes that target characteristic blood vessels associated with tumors. “That means that instead of blindly removing all the lymph nodes, for example, in endometrial cervical cancer cases, we can use imaging and dyes to just take out that one lymph node that lights up and not destroy normal tissue.”
Imaging is just one area that will influence cancer treatment. One thing that intrigues Minsky is the potential melding of radiation and immunotherapy. Study results have suggested that radiation increases the body’s natural antitumor, immunogenic response (called the abscopal response), although scientists still don’t know the exact mechanism behind this. “There’s still a lot of basic science research ongoing, but we find that when we treat the tumor with radiation, the tumor gives off some signal to enhance the body’s immune response, and that is further enhanced by immunotherapy,” he says. Clinical trials are now under way to examine this phenomenon.
Many other advances are in the works that will help to eliminate even the most insidious tumors, Orringer remarks. “It’s an exciting time. And it is also a time to encourage the collaboration between physicians, basic scientists, and engineers. Single-handedly, there’s no way I would have had the opportunity to address the concern that I had back when I was a medical student and seeing my first brain-tumor operation.” He adds, “The progress that we were able to make on the microscope, and all the progress we are making in this area of cancer technology, has come and will come through close cooperation between different disciplines as well as between academia and industry. It’s only through that kind of cooperation that I think we will be able to develop the approaches that are really promising and that will be useful to us in the fight against cancer.”
References
- C. W. Freudiger, W. Min, B. G. Saar, S. Lu, G. R. Holtom, C. He, J. C. Tsai, J. X. Kang, and X. S. Xie, “Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy,” Science, vol. 322, no. 5909, pp. 1857–1861, Dec. 2008.
- C. W. Freudiger, W. Yang, G. R. Holtom, N. Peyghambarian, X. S. Xie, and K. Q. Kieu, “Stimulated Raman scattering microscopy with a robust fibre laser source,” Nature Photonics, vol. 8, no. 2, pp. 153–159, Feb. 2014.
- M. Ji, D. A. Orringer, C. W. Freudiger, S. Ramkissoon, X. Liu, D. Lau, A. J. Golby, I. Norton, M. Hayashi, N. Y. Agar, G. S. Young, C. Spino, S. Santagata, S. Camelo-Piragua, K. L. Ligon, O. Sagher, and X. S. Xie, “Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy.” Science Translational Medicine, vol. 5, no. 201, p. 201ra119, Sept. 2013.
- M. Ji, S. Lewis, S. Camelo-Piragua, S. H. Ramkissoon, M. Snuderl, S. Venneti, A. Fisher-Hubbard, M. Garrard, D. Fu, A. C. Wang, J. A. Heth, C. O. Maher, N. Sanai, T. D. Johnson, C. W. Freudiger, O. Sagher, X. S. Xie, and D. A. Orringer, “Detection of human brain tumor infiltration with quantitative stimulated Raman scattering microscopy,” Science Translational Medicine, vol. 7, no. 309, p. 309ra163, Oct. 2015.