The mosquito is the deadliest animal in the world (Figure 1). It is the main carrier of parasites that cause malaria, which is a bigger killer than any other disease in history; in fact, some blame malaria for the deaths of half the humans who have ever lived. Today, malaria continues to have a devastating effect on the health of millions of people.
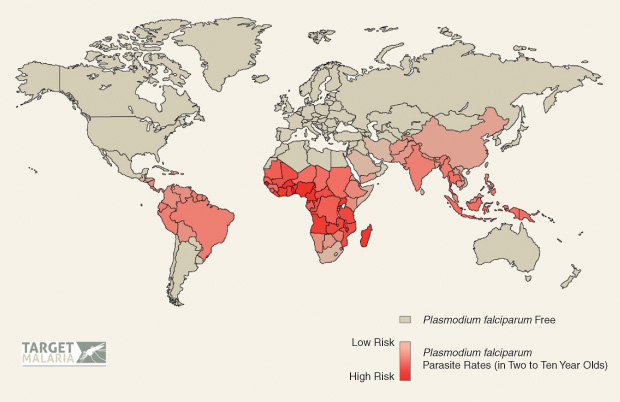
According to the World Health Organization, more than 3 billion people live in areas of high malaria risk (Figure 2). In 2015, the disease caused 438,000 deaths worldwide; more than 70% of these were of children under five—which equates to the death of one child due to malaria every two minutes. Over 90% of these deaths occurred in Africa, where the females of the genus Anopheles gambiae complex pose the greatest risk factor.

Antimalarial drugs have proven effective to a degree; but there is a danger of growing resistance, and problems beset the delivery of drugs to at-risk populations affected by poverty and a lack of infrastructure. The astronomical social and financial costs of malaria have fueled the search for a new approach, and Target Malaria, a research program that began at Imperial College London, may have the answer in a gene drive that could rapidly reduce or potentially eliminate the disease’s major carrier.
Target Malaria Sees a Prolonged Effort

“Our goal is to eliminate malaria,” says Andrea Crisanti (Figure 3, right), a professor of molecular parasitology at Imperial College London, who believes that ridding the world of the disease will require both the gene drive and the ongoing development and distribution of effective drug treatments. “It is always more effective to use more than one method,” he explains. “In recent years, malaria transmission has reduced, so we just need an additional tool to look at eradication. But it requires a prolonged and sustained effort that can be extremely expensive and difficult to achieve. This is well exemplified in the case of polio, which has proven very hard to eradicate.”
In addition, social factors—such as civil unrest or poor infrastructure in, for example, Nigeria and parts of Southeast Asia—can make it difficult to distribute drugs effectively. In the developed world, on the other hand, the greater concerns are over the side effects of vaccines. “Eradication costs thousands of times more than control, and that is why we are getting the mosquitoes to do the work for us,” according to Crisanti.
Crisanti is in charge of experimental activities in the laboratory. His colleague, Prof. Austin Burt, an evolutionary theorist who specializes in selfish-gene research, coordinates the project, and Dr. Tony Nolan oversees the laboratory work. Together, they aim to reduce the number of female A. gambiae mosquitoes by targeting genes that produce nucleases which cut specific DNA sequences.
By reprogramming endonucleases to act like homing endonuclease genes—nuclease genes that copy themselves from one chromosome to another—they can interrupt the genes’ key to fertility or pathogen transmission so they cease to function. The modified malaria mosquitoes would then pass on these genes to a disproportionately high percentage of their offspring and quickly spread the modification throughout a specific population. Two key strategies are biasing the sex ratio of mosquito populations and reducing female fertility.
“The project has a long history—ten years—and it builds on three pieces of science: 1) the genetic modification for mosquitoes that my laboratory developed, 2) Burt’s idea to exploit nucleases as a tool to create a synthetic gene-drive system to pass on genes to offspring, and 3) CRISPR,” says Crisanti. He goes on to explain, “Clustered regularly interspaced short palindromic repeats—CRISPR—is more flexible and more specific than previous tools, which used nucleases to generate a gene drive but were difficult to engineer. CRISPR expedites the process and shortens timelines, so it is a game changer as an enabl ing technology.”
Technology Outpaces Regulation
CRISPR is a relatively new genome-editing tool that is faster, less expensive, and more accurate than previous techniques, and it has allowed Target Malaria to further its research more rapidly. Now, the regulatory landscape has to catch up with the technology.
“Before CRISPR, there were a few major roadblocks,” notes Crisanti. “The first was cumbersome engineerability, which we have now solved. The second was the lack of a clear regulatory framework; but the issues have been highlighted, and guidelines are now being drafted.” He continues: “The problem, however, is that all regulations on genetic modification relate to plants. That is a very different scenario because, at one end, you have a private entity driven by profit, and, at the other end, you have a community that wants safety and species diversity. The aim is a zero-sum profit but no environmental impact.”
Target Malaria sits outside current regulatory standards because it is not driven by profit. Legislation must, therefore, take into account the risks and benefits of a technology that deals with animals, is intended to spread, and seeks improved health rather than profit as the key outcome.
The project has certainly attracted many backers, and its core grant comes from the Foundation for the National Institutes of Health through a program of the Bill and Melinda Gates Foundation, which has, so far, contributed nearly US$100 million. It also has support from scientists, stakeholder-engagement teams, risk-assessment specialists, and regulatory experts in Africa, North America, and Europe. With this support, the team is fully focused on the science and firmly believes it can soon overcome the remaining engineering hurdles.
“We have very generous funding for the next three-and-a-half years to deliver a field-ready product,” reports Crisanti. “If the regulatory and safety requirements are fulfilled, then it could be used. We are making faster progress because proof of principle has been demonstrated before, and CRISPR came at the right time.” He adds, “Now, we have to improve effectiveness and go beyond proof of principle. We must also look at off-target effects from the manipulation of genes. Nucleases are not a magic bullet, so we must build a matrix to judge their effects. Also, we have to minimize the possible mutation in DNA that could give rise to resistance.”
“I don’t see these as roadblocks,” Crisanti asserts. “They are just problems that we need to solve. The current priority is the off-target effect.” He adds, “We need to avoid any unwanted phenotypes through the effect on other areas of DNA that could, for instance, undermine the fitness of the mosquito.”
Implementation is Key
The final hurdle will be logistics. A gene drive needs a large number of mosquitoes and a proper release, which would initially happen in a geographically confined area so that the team can understand how mathematical modeling compares to reality.
Crisanti says, “We need to get the number of mosquitoes right as well as the place and the density of release. After that initial phase and some capacity building, we could look at a countrywide release. Now that we have CRISPR, we are confident we can meet our deadline.”



