Three hundred eighty-two million people in the world have diabetes today. Of those, roughly 343.8 million have type 2 diabetes—the variant associated with obesity and insulin resistance—3.8 million have type 1, and 175 million don’t even know they have diabetes at all. On top of that, 316 million more people are at high risk for diabetes. Combined, that makes for just shy of 10% of the global population with or at risk for the disease right now.
Many of these cases are in the notoriously overfed and under-exercised developed world, such as the United States (24.4 million with diabetes) and Europe (56.3 million). But far more are in the lower- and middle-income countries, where maturing economies, westernization, and biological predispositions have created a diabetes pandemic of epic proportions. In 1980, less than 1% of China’s population had diabetes; today, it is the global epicenter for the disease with 114 million people who have diabetes—11.6% of the populace. India has 65.1 million people with diabetes, up from 50.8 million in 2010. These countries are the rule, not the exception: according to the International Diabetes Foundation (IDF), which crafted these estimates, 80% of the total global diabetes population currently lives in lower- and middle-income countries.
At its simplest, diabetes is a dysfunction in a person’s production or efficacy of insulin, the hormone made by the beta cells of the pancreatic islets that is responsible for shuttling glucose out of the bloodstream and into the rest of the body. There are two primary variations: In type 1 diabetes, the body destroys those beta cells, forcing the patient to self-inject insulin in compensation. In type 2 diabetes, the body loses its ability to respond to the hormone, a state called insulin resistance; in advanced stages, the beta cells can also essentially burn out, requiring these patients to also supplement with insulin. In both cases, diabetics are at risk for long-term damage to nerves, organs, and blood vessels as a result of high blood sugar, or hyperglycemia. It is a leading cause of blindness, renal failure, and amputation, and roughly 50–80% of patients with diabetes die of cardiovascular disease.
The crisis point for diabetes has frankly come and gone. In 2006, the IDF estimates did not have us near the 380 million mark for another ten years, yet we are now on track to have 592 million people in the world with diabetes by 2035, with another 471 million at risk. That is slightly more than the current combined populations of the United States, Russia, Brazil, Indonesia, and Japan.
Five point one million people died last year as a result of their diabetes. Dialysis centers, facing an unprecedented influx of renal failure patients, have become a multibillion-dollar global industry. In Santa Barbara, California, where Kristin Castorino works as a research physician at the Sansum Diabetes Research Institute, two new mothers in their 20s have lost their eyesight postpartum to type 2 diabetes. “And that’s what we’re going to see,” Castorino says. “That is how devastating diabetes is.”
The Rise of the Machines
The earliest reports of diabetes go back to the first century A.D.—“a melting down of the flesh and limbs into urine,” is how Greek physician Aretaeus described it—but until the 20th century, it was a rare and generally fatal disease. The breakthrough came in 1921, when Frederick Banting and his colleagues at the University of Toronto, Canada, discovered insulin. The next year, the first human patient was a 14-year-old boy named Leonard Thompson, who was nearly dead from diabetes at the time. The treatment dropped the boy’s blood sugar levels so dramatically that he recovered well enough to live for another 13 years—an unprecedented new lease on life for a type 1 diabetic at the time. The demand for the drug took off immediately, and, by the end of 1923, the pharmaceutical company Lilly was mass-producing the drug and an estimated 7,500 physicians had already treated 25,000 diabetic patients.
The introduction of insulin threw the doors open to new discoveries. First among these was Banting’s insulin treatment. But while it was a Nobel Prize-winning therapy, it was not a true cure for the disease. Patients did live longer, but they didn’t necessarily live better. As patients now could survive for decades, the long-term effects of chronic hyperglycemia began to surface—blindness and gangrene, for example. It was the start of the era of complications. Doctors also began to realize that diabetes patients could be divided into two categories: those who responded and were absolutely dependent on insulin, and those—typically heavier, older patients—who were not. At first, these categories would be distinguished as juvenile- and adult-onset diabetes, but, as prevalence rates for both types began to blur age boundaries and more information about diabetes emerged, they were designated types 1 and 2.
All the same, by the time the 1970s and 1980s rolled around, the entire matter appeared to be on the verge of resolution. New types of insulin had been developed that were safer and cleaner, and blood-sugar regulating drugs had emerged for type 2 patients. The outlook on type 1 diabetes was particularly rosy: Researchers had finally established that it was an autoimmune disease and found various auto-antibodies in the blood of diabetics that could begin to serve as predictors for the disease. Others had discovered a genetic link in the region for human leukocyte antigens (HLAs), bred two animal models [the nonobese diabetic (NOD) mouse and the biobreeding rat], and began experimenting with islet cell transplants. A cure, expresses Mark Atkinson, codirector of the University of Florida Health Diabetes Center of Excellence (Figure 1), seemed imminent. “When I started out in this field 30 years ago,” he recalls, “I thought a career in type 1 diabetes would be short. So many people did because we had everything we needed.”

Today, that optimism might seem misplaced. More people in the world have diabetes right now than have ever contracted HIV. There is no cure for type 1, and the situation for type 2 is very urgent indeed. But still, that period in the 1970s marked a time of extraordinary energy in the field, which paved the way to how we think about and approach the disease today. Thanks to two landmark studies developed during that time and published in the 1990s and early 2000s, the new goals of diabetes care are to keep blood sugar as close to normal as possible for all types and, for at-risk type 2 patients, to stop the disease from developing at all. The first aim comes courtesy of the 1993 Diabetes Control and Complications Trial, which gave 1,441 type 1 diabetics either the standard glucose control guidelines of the day or intensive control maintained as close to normal as possible via multiple daily glucose tests and insulin doses, assessed by a blood marker called HbA1c, or glycosylated hemoglobin. At the end of a decade, the tight control group’s risk for eye, kidney, and nerve disease had plummeted in comparison—by up to 76% in the case of eye disease. Follow-up work showed that the effect carried through to a 42% drop in their risk for cardiovascular disease.
“That’s the absolutely huge thing that’s happened in the last 30 years,” says John Pickup, a diabetes researcher at King’s College London. “Tremendous effort has been put into improving diabetes control and getting as close to normal as possible. That’s really the incentive for new ways of treating diabetes, and new ways of monitoring it—you start from that position.”
The second major effort was the Diabetes Prevention Program (DPP), a 27-center study that reported in 2002 that regular exercise—aiming at 150 min a week—and a modest 7% weight loss through diet could prevent at-risk patients from developing type 2 diabetes by 58%. This effect was even more pronounced in patients over 60 who saw their disease risk drop by 71%. Lifestyle change has also been shown to help moderate and control type 2 diabetes and its complications.
Helping patients reach their blood sugar goals is an active industry of technologies and pharmaceuticals that began gradually in the 1960s and 1970s and then flourished after the turn of the century to fill the space where the diabetes cure failed to manifest. These days, modern diabetics can watch their blood sugar rise and fall in real time using continuous glucose monitors that tap into the body through sensors that sit just under the skin. They can administer insulin through sophisticated pumps that similarly attach to the body and infuse the hormone in via tiny tubes or even discrete patches that connect wirelessly to a nearby control device. Most of these devices need changing only every two or three days, and all are portable, weighing no more than a few ounces at most. Some pump software can help diabetics calculate the amount of insulin they should take at meals and remind them to check their blood sugar and change the insulin cartridge. So far, these technologies have been used mostly for type 1 diabetics, but their effectiveness in advanced type 2 patients, whose beta cells require increasing insulin support as their disease progresses, is under investigation, and health authorities in several countries cover insulin pumps.
The insulin these technologies deliver is also better as most of it is pure bacteria-produced formulas instead of the earlier types of animal-derived formulas. New variations also vary in effect, from long to intermediate to fast-acting, so that patients can stack and combine different types to better recreate the low basal and spiking mealtime patterns of a healthy pancreas. Type 2 patients have benefited particularly from the arrival of myriad types of blood-stabilizing pharmaceuticals that help increase insulin sensitivity in the liver, stimulate the pancreas to release more insulin, or improve insulin’s effect in the muscles. A new class of drugs (sodium-glucose cotransporter 2 inhibitors) has just been approved by the U.S. Food and Drug Administration and is noted for its ability to encourage the body to dump extra glucose out through urine.
Collectively, the past half-century’s developments have brought incredible improvements to the patient with diabetes. Life expectancy for a type 1 diabetic diagnosed between 1965 and 1980 is up 15 years compared to those born between 1950 and 1964 and is now less than four years behind the general population. Early childhood deaths are down for type 1 patients, as are complications such as kidney failure and nerve damage. With tools like the insulin pumps that better mimic a genuine pancreas, some with diabetes can even enjoy the luxury of occasionally sleeping in, missing a meal, or just eating when they are hungry instead of responding immediately when their blood sugar dictates. Thanks to the DPP, we also know how to stop type 2 diabetes from developing in at-risk patients. We know the weight goals to aim for, the minutes of physical exercise to recommend, and the foods to emphasize.
A Formidable Opponent
However, none of this is enough. Despite these advances, diabetes is still increasing and millions are still dying. The spread of type 2 diabetes remains frustratingly out of control and, quite frankly, lifestyle interventions haven’t made a dent in its global progression. Type 1 diabetes is still far from a cure, and gadgets like insulin pumps are too expensive for many. Even when the patient can afford such tools, they are still beholden to their disease. “I resent that self-tracking erodes the invisibility of my disease,” writes type 1 diabetic Kim Vlasnik on her diabetes blog, Texting My Pancreas. “An insulin pump and continuous glucose monitoring (CGM) provide me very valuable data, but they also mean that I am never simply me, physically.”
The problem is we don’t have all of the information we need. We do not, for instance, know what causes type 1 diabetes or why it has risen at a steady pace of 3–5% since the mid-20th century. Those variants in the HLA genes have turned out to explain about 60% of a person’s genetic susceptibility to type 1 diabetes; several dozen different loci make up the difference, and scientists are still working out how they contribute. As it is, though, only 15% of type 1 cases have a first-degree relative link, and not all type 1 patients have the HLA-gene risks either, so environmental factors clearly play a role, but which factors and how? Investigations have explored everything from cow’s milk to wheat gluten to inadequate immune exposure (the hygiene hypothesis) and various viral triggers, but, while sometimes promising, none have produced any definitive trigger. It is altogether likely that there are many different causes for diabetes that vary from region to region.
Much of our current understanding of type 1 diabetes’ onset and progression stems either from pathology studies done in the 1960s or from more recent work done in animal models like the NOD mouse. But for the most part, as is so often the case in animal models, those findings simply haven’t panned out the way researchers thought they would. Type 1 diabetes has already been successfully prevented in NOD mice many times using—by 2005 alone—192 different methods, including techniques as mundane as varying the shelf level of the mouse’s cage. But there is still no way to prevent it in humans. Diabetes, as Mark Atkinson says, “has turned out to be a formidable research opponent.”
The state of knowledge around type 2 diabetes isn’t that much better. On the one hand, we have made enormous progress in understanding some of the biological players involved in the disease. The traditional view once included essentially the liver making glucose, the fat storing energy, the muscle storing and using energy, and the pancreas orchestrating it all. But over the years, that model has stretched to encompass a wealth of new information, such as the role of the brain and various gastrointestinal tract products such as bile acids and hormones like glucagon-like peptide-1, which increase insulin secretion and quell the production of new glucose from the liver. This is why so many drug classes have emerged in the last couple of decades to help with type 2.
But we’re missing crucial fundamentals like why it’s rising so fast. We do know that type 2 diabetes is associated with the spreading sedentary western lifestyle and obesity, but that relationship isn’t absolute. Seventy-five to 80% of obese individuals never develop the disease, while certain ethnicities, like east Asians, can develop diabetes with minor weight gain and a body mass index that a western doctor would call healthy. It is still not clear what the trigger is that kicks off the insulin resistance in the first place. Some researchers surmise that it may be a malfunction in the way fat cells grow. Either way, it is increasingly apparent that these patients are predisposed to the disease at birth, but, again, that exact predisposition remains likewise unclear. Type 2 diabetes has more than 50 genes associated with it so far, but, altogether, they still explain just 10% of the genetic cause, and, says Steven Kahn, who is at the VA Puget Sound Health Care System and is the director of the University of Washington Diabetes Research Center, “we still haven’t come up with any major therapeutic target that’s built on what the genes do.”
Then there’s the fact that we’re still not preventing diabetes very well, despite the DPP. Implementing lifestyle changes in the United States, Europe, and other developed nations has proven hard enough, but it’s much more difficult in the countries that need it the most, including India, China, and the Middle East. “Many of the developing countries, they are caught in this big surprise,” explains William Hsu, senior director of Joslin Health Solutions International at Joslin Diabetes Center. “They were dealing with the scourge of infectious disease. That has just left their doorstep, and, all of a sudden, they’re facing these noncommunicable diseases.” Their health care systems, Hsu adds, are just not set up for the complex, continuous, and expensive care that a chronic disease demands. Many patients are not even aware of diabetes, and, even when they are, more pressing problems often take priority, such as safety, poverty, and war. It should come as no surprise perhaps that patients in these countries have more than twice the mortality rate of their developed-world counterparts, and are much more likely to suffer kidney and eye disease and stroke. In Egypt, the rate of retinopathy in newly diagnosed diabetics is 15.7% compared to Australia’s 6.2%.
Adding to the handicap these regions face is the increasingly evident role that epigenetic factors play in promoting the diabetes cycle. Several lines of research show that if a mother is either malnourished or overnourished during pregnancy, the child is more likely to develop type 2 diabetes later in life. The Dutch Hunger Winter is the most famous example of this: In the winter of 1944 to 1945, The Netherlands was in a state of starvation. The infants who were exposed to the famine while their mothers were in their third trimester were smaller than their well-fed counterparts, and, as adults, they were prone to glucose intolerance and diabetes. Epigenetic analysis found that they also showed a different pattern of methylation on the gene for a key growth protein called insulin-like growth factor-2, suggesting that their expression of this gene was different from their peers in a manner distinct from their inherited genetic code. A similar priming effect occurs for children born to mothers with gestational diabetes and hyperglycemia, and the mothers themselves are also more likely to develop type 2 diabetes after gestational diabetes.
As of now, gestational diabetes is on the rise worldwide just like type 2, interspersed with pockets of starvation in places like Syria, the Central African Republic, and Afghanistan. Because of this, as well as the continuing spread of the western lifestyle and environmental factors that we are not yet aware of, diabetes is going to skyrocket in these countries in the next 20 years. In the western Pacific region, which already has the highest diabetes prevalence in the world at 138.2 million, diabetes will increase by 46%. Southeast Asia will see a rise of 71%, the Middle East and north Africa 96%, and south and central Africa 109%. Diabetes strikes earlier in life in these regions than in the west, by at least a decade on average, so many of these patients will be under 60—still their families’ breadwinners and their countries’ workforce. “This disease,” says Hsu, “has the potential to bankrupt a society.”
Gathering Strength
If all of this sounds grim, it is. But there are also reasons to hope. We’re in a period of greater cooperation and innovation now than has ever been the case in diabetes. Efforts to stem the flood of patients are underway in countries all over the world, which, given that the United Nations General Assembly didn’t even recognize diabetes as an international public health challenge until 2006, is progress. In 2007, the IDF kicked off a funding program called Bringing Research in Diabetes to Global Environments and Systems (BRIDGES), which aims to identify ways to effectively implement diabetes interventions around the world in a lasting way. So far, the organization has supported 38 projects in 34 different countries, including lifestyle changes led by locals in Venezuela, microclinics supported by family and friend networks in Jordan, and strategies that brought free HbA1c screenings to sub-Saharan Africa and saw patients’ levels drop by a full percent over the course of the year in return. Multiple BRIDGES projects also target gestational diabetes in Canada, the United Kingdom, China, and India. Researchers in these programs are trying to screen and educate women on the risks that high blood sugar poses to their children and themselves and guide them into healthy glycemia and weight gain levels during and after pregnancy. Success has been mixed so far, but some of the work is still under way.
Telehealth and mobile health strategies are still in development but have enormous potential to become key components to sustainable care in remote areas. “The number of mobile lines here in Egypt is more than the total number of the population; they can reach to every far corner of the country,” says Adel El-Sayed, chair of the diabetes unit at Sohag University in Egypt and of the Middle East and north Africa region of the IDF. He is collaborating with cell phone companies to find ways that might work against diabetes in his country, whether by delivering health messages or perhaps following up medical care between doctors and patients.
At the Madras Diabetes Research Foundation in India, researchers have already found some success in introducing telemedicine screening and health care to rural patients in southeastern India (see “Telemedicine in Diabetes Care” by Mohan and Pradeepa in this issue of IEEE Pulse). In the developed world, places like the renowned Joslin Diabetes Center and the diabetes management company Glooko are working on ways to harness the mobile care data already in existence from phones, insulin pumps, glucose monitors, fitness trackers, and so on. “As we look at the global pandemic of diabetes and risk of diabetes, our thinking is, look, we have to approach this very differently,” explains John Brooks, Joslin president and CEO. “There’s so much information—how do you make sense of it all? Our thinking is: let’s get all those data points together, apply our Joslin expertise to create some predictive algorithms, and distill that sea of data into very straightforward, simple, clinically relevant recommendations.”
Meanwhile, the broader diabetes technology sector has only grown more sophisticated. Researchers have begun to combine insulin pumps with continuous glucose monitors to create what’s called an artificial pancreas, which is capable of automatically monitoring and responding to blood sugar changes without the patient (see “Biosensors in Diabetes” by Renaud et al. in this issue of IEEE Pulse). Prototype versions have already appeared in different labs and clinical trials of various sizes across the United States, Europe, and Australia. The pharmaceutical company Medtronic is among the furthest ahead with an insulin pump-continuous glucose monitor called the MiniMed 530G with Enlite already approved in the United States (Europe approved a similar device in 2009). The device can’t control blood sugar precisely yet, but it can suspend insulin delivery when blood sugar gets too low, such as at night, when a patient might not realize that they are slipping into hypoglycemia.
Islet cell transplantation, which has been around since the 1980s but has lately been on the rise, is also poised for change. The technique has the potential to give someone full independence from insulin, but the effect tends to last for only a few years and requires long-term immunosuppression. One solution, called encapsulation, involves encasing the transplanted islet cells in protective polymers, like a high-tech tea bag that sits in the body and lets insulin out but keeps the immune system at bay. Until recently, such encased islets tended to fail after the body built up layers of scar tissue around them, but a new research effort led by JDRF (formerly the Juvenile Diabetes Research Foundation) has spurred a systematic search for more biocompatible materials that wouldn’t cause such a reaction.
Even the knowledge front is advancing into new ground, thanks to projects like the Network for Pancreatic Organ Donors with Diabetes (nPOD). Pancreas tissue is difficult to sample in the best of times, and donors are rare, which has made large-scale studies of human diabetes a challenge at the cellular level—hence the field’s long-standing reliance on animal studies to understand the disease. The nPOD program, another JDRF project, gathers diabetic pancreases from donors across the United States and ships the tissue out to different researchers for study. It’s only seven years old at this point but has already sent some 30,000 samples to more than 120 labs around the world and helped reveal crucial information gaps.
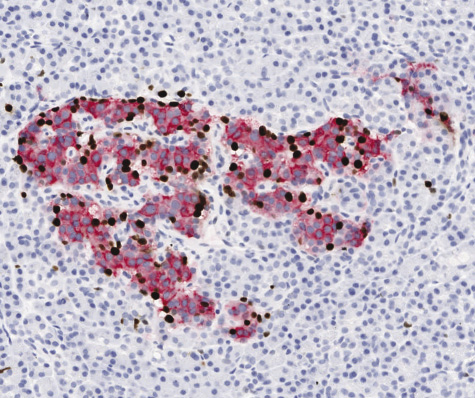
There’s the fact that, for instance, some long-standing diabetic pancreases still have beta cells in their islets and that differences in cell patterns might mean that there are at least two different types of type 1 diabetes, if not more. The hallmark of diabetes development in the NOD mouse is a massive infiltration of immune cells into the pancreas’s islets, and it was long assumed that this happened in human cases as well. But as studies of nPOD tissue have shown, that’s not the case at all, which may be at least part of the reason why treatments have translated so poorly from the preclinical to the clinical, says Atkinson, who is nPOD’s executive director (Figure 2). “We’ve been preparing for battle like we’re fighting against a blitzkrieg by the immune system,” he admits. “But what we’re seeing in humans is that it appears to be more like a sniper attack.” Many in the field have high hopes for the program’s long-term impact. “This is probably the best thing that has happened for understanding the pathology of the disease for a long time,” says Matthias von Herrath, who directs the Center for Type 1 Diabetes Research at the La Jolla Institute for Allergy & Immunology and has used nPOD tissue in some of his work. “I think from these studies we will gain, in the next decade or so, the best insight into the basic causes of the disease.”
None of this will be a cure-all, but combined it could be enough to start making at least a dent. The real change will come if and when the world can bring its combined weight to the fight at every level, from the individual to the societal and even the legislative. “Medicine alone cannot solve this problem,” says Hsu. He likens the situation to smoking and the transition of that activity from popularity to pariah. “Did taxing cigarettes do the job alone? No. Did it help? Sure. Was outlawing smoking in public places the answer? No, but it helped,” he says. “The diabetes problem cannot be solved through a single channel. The engagement has to be across the board, from every level, on the private side, on the public side, on the academic side. We’re talking about a full-frontal coalition for us to really tackle one of the biggest health care challenges facing humanity.”
That effort simply has to be made, and it’s going to succeed because, if it doesn’t, more than a billion people will either have diabetes or be on their way to it in the space of a generation. “And that just can’t happen,” adds Joslin’s John Brooks. “We can’t let that happen.”