Above: Figure 1: iPSCs reprogrammed from a woman’s skin. (LEFT) The nuclei are shown in blue; green and red indicate proteins found in reprogrammed cells, but not in skin cells. (RIGHT) iPSCs such as these can be matured and used to study models of disease. (Images courtesy of the laboratory of Kathrin Plath at the University of California, Los Angeles.)
Interest in stem cells escalated in 2006 when scientists figured out how to reprogram some specialized adult cells to assume a stem-cell-like state. Called induced pluripotent stem cells (iPSCs), these cells opened the door to a range of potential applications, including generating cells and tissues to replace those that are faulty or missing in patients with cancer, diabetes, cardiovascular disease, or other maladies (Figure 1, above). Visions of new treatments and even cures for debilitating and fatal illnesses proliferated, and some of that work is well under way (see “A Wealth of Research”). Now, ten years later, those visions are looking more like real possibilities as research moves from the lab to the clinic and expands toward a greater understanding of the basic science behind stem cells and its applications.
[accordion title=”A Wealth of Research”]
Novel technology is yielding scaffolds of neurons built from adult tissue-derived stem cells [1], another technique using stem-cell therapies is being developed to repair injured tissue [2], and a new type of stem cell with possible applications for regenerative medicine has been discovered [3]. This is just a tiny sampling of the stem-cell work published or announced in 2016. Clearly, stem cells have become a prominent area of study, and many believe they will soon provide a wealth of valuable clinical options for patients struggling with disease. That includes prominent researchers Khalid Shah, principal faculty with the Harvard Stem Cell Institute and director of the Stem Cell Therapeutics and Imaging Program at Massachusetts General Hospital, and Dieter Egli, assistant professor of stem-cell biology at Columbia University Medical Center in New York.
Tackling Brain Tumors
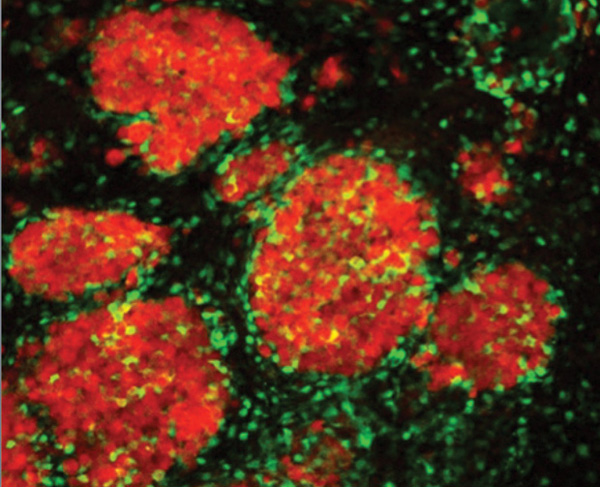
The fight against brain tumors is one of many areas that may harvest significant benefits from stem-cell therapies, according to Shah. “The problem is that brain tumors are difficult to treat because systemic drugs that work on breast, lung, and other types of cancer do not reach tumors in the brain (because of the blood–brain barrier). So we’re using a different approach: we’re engineering stem cells with therapeutics that can be delivered very locally to both primary and metastatic brain tumors, can release the therapeutics at the site of the tumor, and can kill the tumor cells,” he says [4], [5].
This involved multiple challenges. One was developing a tumorkilling agent. For this, Shah’s group developed stem cells that either release tailor-made therapeutic proteins or oncolytic viruses best suited to kill an individual patient’s particular tumor cells. “Any time you’re developing first-line therapies, you need to understand the disease very well and to know exactly what you’re targeting on the tumor cells, because different patients have different signatures on their tumor cells,” he explains. This meant Shah’s group had to collect a large cohort of patient tumor lines, which not only included cell lines from the brain tumors themselves, but also from breast cancer, melanoma, and other cancers that often metastasize to the brain. Armed with that mass of gathered information, they specifically designed stem cells carrying receptor-targeted therapeutic proteins and oncolytic viruses to home in on tumor-cell targets (Figure S1).
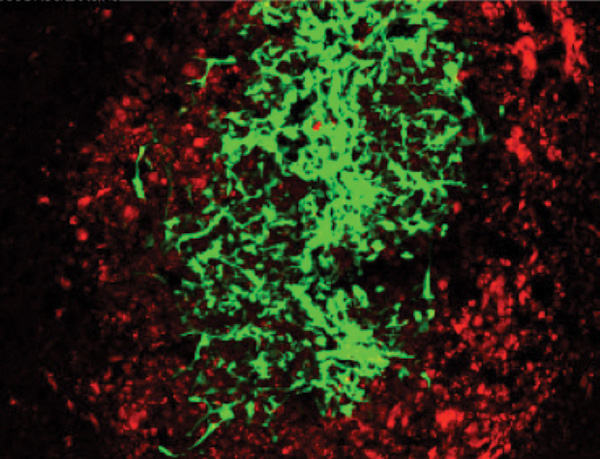
The other major challenge was getting the therapeutic-carrying stem cells to the tumors in the brain. The group’s answer was to encapsulate the stem cells inside biocompatible gel (Figure S2). This way, the stem cells could be implanted into the tumor resection cavity created after the primary tumor was removed from the brain. For metastatic tumors that form multiple tumor deposits in the brain and where surgery is not an option, Shah’s group injected the stem cells into the bloodstream in the artery of the neck to reach the site of the tumors in the brain. Once at the site of the tumor, the stem cells slowly released either the targeted therapeutic proteins or oncolytic virus and killed the tumor cells. Recently, Shah’s group has been able to optimize the procedure using stem cells engineered with different targeted therapeutic proteins and oncolytic viruses to treat tumors in the brain. Shah has now submitted applications to the U.S. Food and Drug Administration (FDA), anticipates FDA approval, and hopes to advance toward clinical trials within a few years.
Replacing Beta Cells

Egli and his research group (Figures S3 and S4) are focusing on beta cells, which store and release insulin to keep blood glucose levels in check. “In diabetes, however, beta cells are either lost or they are no longer functional, and the body cannot replace them,” Egli explains. This is where stem cells come in. His group’s objective is to take cells from an adult patient who has type 1 diabetes, reprogram the cells to a pluripotent state, and direct them to make replacement beta cells for the patient, thereby curing the disease.
Currently, Egli’s diabetes research is building the foundation necessary for clinical application. “There is a lot of work that has to be done in preparation for that, and that includes testing stem cells in culture to understand how cells work when they do work and how they fail when they don’t,” he says. His group has already shown that it can derive stem cells from embryos cloned from a patient with type 1 diabetes, which can be differentiated to beta cells that produce insulin in response to glucose levels [S6]. “If these human cells can protect an animal from diabetes, it’s more likely that it will also work in humans, so we expect this should be the case,” Egli contends. A key part of this work was the ability to generate stem cells using human oocytes. Egli led the team that in 2014 reported its creation of the first disease-specific embryonic stem-cell line with two sets of chromosomes using somatic cell nuclear transfer (SCNT). SCNT is a technique that implants a donor nucleus into an enucleated oocyte. “This unique technique has allowed us to make stem cells that are genetically matched to a patient,” he says, explaining that they used this method to produce beta cells specifically for an individual diabetic patient. Because they are derived from the patient’s own stem cells, such patient-matched stem cells forgo the alloimmunity issues other stem cells would have. “These patient-matched stem cells are made on a one-by-one basis, so there are economic challenges to this approach—it would be more costly than making one cell line and trying to fit it to everybody—but ultimately, if people have the choice, I think they would prefer using their own stem cells to cure them.”
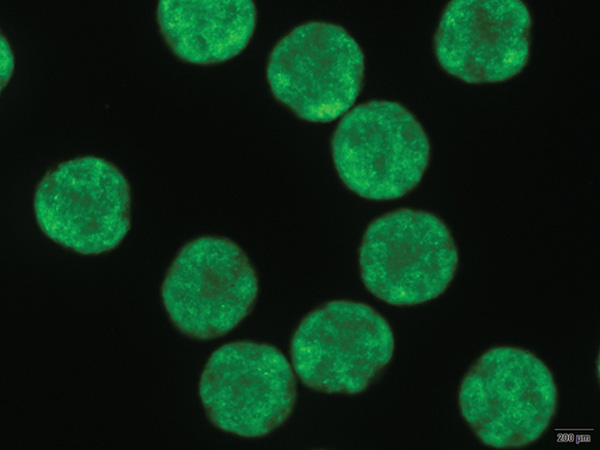
Egli and his lab are now using their stem-cell approach to take a closer look at the disease of diabetes, particularly why the diabetic immune system attacks beta cells in the first place and how to turn off that cell-destroying immune response. They are also conducting in-depth studies of derived beta cells using cell lines from many different patients. This will provide more insight into even slight differences between their derived cells made in culture and naturally occurring cells made by the body.
Other labs are now beginning the task of eventually transferring the basic research on potential stem-cell therapies into clinical therapies, and Egli isn’t sure how long that will take. He is convinced, however, that stem cells will be a part of health care in the future. “I’m not the only one saying that stem cells can be a cure for diabetes or for a number of different diseases, such as Parkinson’s disease, heart disease, and others, where a patient’s cells are missing or dysfunctional,” he asserts. While those therapies may be a few years off, Egli has no doubt they are coming. He adds, “There are significant projects under way here and elsewhere that make me very confident that stem cells will ultimately fundamentally change how we approach diseases.”
References
- Rutgers University. (2016, Mar. 17). Future brain therapies for Parkinson’s possible with stem cell bioengineering innovation. [Online].
- Phys.org. (2016, Apr. 4). Scientists develop “game changing” stem cell repair system. [Online].
- L. Cameron, T. Parenti, and A. Ralston. (2016, Mar. 3). MSU discovers a new kind of stem cell. MSU Today. [Online].
- T . Bagci-Onder, W. Du, J.-L. Figueiredo, J. Martinez-Quintanilla, and K. Shah, “Targeting breast to brain metastatic tumours with death receptor ligand expressing therapeutic stem cells,” Brain, vol. 138, part 6, pp. 1710–1721, June 2015.
- M. Duebgen, J. Martinez-Quintanilla, K. Tamura, S. Hingtgen, N. Redjal, H. Wakimoto, and K. Shah. (2014, May). Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. [Online].
- M. Yamada, B. Johannesson, I. Sagi, L. Burnett, D. H. Kort, W. Prosser, D. Paull, M. Nestor, M. Freeby, E. Greenberg, R. S. Goland, R. L. Leibel, S. L. Solomon, N. Benvenisty, N., M. Sauer, and D. Egli, “Human ooyctes reprogram adult somatic cells to diploid pluripotent stem cells,” Nature, vol. 510, no. 7506, pp. 533–536, June 2014.
[/accordion]
The Human Focus
One of the major changes over the past few years has been the increasing amount of work centering on human stem cells rather than those of animals (which has been the predominant focus for many years). Part of that reliance on animal stem cells hearkens back to federal-funding restrictions on research using human embryonic stem cells implemented in 2001 [1]. Although those restrictions were lifted in 2009, their effects lingered, according to Kelly Shepard and Kent Fitzgerald (Figure 2), science program officers at the California Institute for Regenerative Medicine (CIRM), the largest source for funding stem-cell research outside the National Institutes of Health [2]. “Even for several years afterward, many people were insecure,” Shepard says, noting that researchers harbored worries that a fickle political climate could potentially constrain funding again.

“As I’ve gone to meetings nationally and internationally on stem-cell biology, it still surprises me a bit how much more of the work in California compared to other places is focused on human stem-cell biology (rather than that of experimental animals),” Shepard remarks. She attributes that concentration of human work to the state’s citizens, who passed a ballot proposal to fund stem-cell work and establish CIRM in 2004. That gave CIRM a stable—and nonfederal— funding source for distribution to research labs doing human stem-cell studies. “As a result, a lot of the CIRM emphasis now is on understanding the differentiation of human pluripotent stem cells. That, in turn, has given us a great deal of insight because there are, in many cases, significant differences between humans versus mice and other mammals,” she says. That’s true even in evolutionarily conserved pathways, such as those associated with certain transcription factors. Shepard explains, “For instance, some transcription factors may act on similar genes in mice and humans, but the timing and location of their expression can be very different, as can the specific activities that they regulate. In other words, a molecule that’s marked or defined for stem-cell populations that can achieve regeneration in a mouse isn’t necessarily a clue that the same cell population exists in humans, so often discoveries that are made in mice have to be confirmed in humans before they can move forward and before they can go down the path toward clinical trial.”
She certainly appreciates the critical contributions of animal stem-cell experiments but notes that human work is also vital to drive the field. For instance, research has shown that mouse embryonic stem cells can be inserted into donor-embryo precursors and implanted into a female, which can then produce viable pups. This approach has been used to test the contribution of embryonic stem cells in the development of an animal and is considered a gold standard for confirming pluripotency. Of course, the same experiments cannot be performed in humans, but Shepard says engineers and other researchers are developing new technological tools to “assess pluripotency in human cells since we don’t have that same kind of gold standard assay we have for mice. This is important as we look at pushing human embryonic [cells] or iPSCs into more of a naïve state, which we think might be able to give rise to more authentic cells and tissues that more closely mimic those you would actually find in the human body.”
Technology Trends
In their roles designing funding opportunities and observing peer reviews, Shepard and Fitzgerald have a front-row seat to the evolution of stem-cell research. This gives them a global perspective and the opportunity to stay on top of the wide swath of up-and-coming technologies and how quickly they infiltrate the multitude of project proposals coming through CIRM.
One new technology having a big impact is the genome-editing tool known as clustered regularly interspaced short palindromic repeats (CRISPR). CRISPR is actually part of the bacterial immune mechanism—it remembers dangerous viruses by keeping a library of telltale viral DNA snippets on hand, and when it uses that library to spot an invading virus, it produces and sends out CRISPR-associated enzymes (especially one known as Cas9) to the site of that DNA snippet, where the enzymes mete out very precise and deadly cuts that eradicate the virus. In 2012, a research team revealed how to commandeer this system [3] as a gene-editing tool that recognizes target DNA sequences with extreme accuracy, snips them out, and replaces them with the desired sequences.
The response from the stem-cell community to the introduction of CRISPR was swift, Fitzgerald says. “People started using CRISPR for all sorts of things. For instance, researchers have been actively looking at taking patient-derived cells, such as those from a Huntington’s patient, and then editing out the mutation and looking to see in vitro whether they can recover the normal phenotype of the cell.” Researchers have also been using CRISPR to determine protein functions: they engineer a mutation, swap the original gene for the mutated one in a subset of cells in a cell line, and then weigh the function of altered cells against that of the unaltered cells, Shepard says. “This gives you a perfect control, because the only thing that’s different is the engineered mutation.” In the future, Shepard continues, CRISPR might even be used to review the genome of an embryo and edit out genetic diseases or as a means of delivering gene therapy to specific tissues in patients with inherited diseases. “Even though I think we’re years away from seeing CRISPR used clinically for gene editing, it’s not so far away that people aren’t already contemplating the possibilities and discussing the ethical implications.”
Single-cell analysis is another very useful technique. Although it is only now starting to make its way into laboratories, it is already providing a previously unseen view of stem cells, according to Shepard. Because this provides data sets on individual cells rather than the population as a whole, researchers now know that iPSC populations have much more variety than assumed, which has led researchers to consider the implications of that heterogeneity, as well as how it came about. “Sorting out the sources of the heterogeneity—whether it’s from the genetic background of the person who donated the cell or from variation in the procedures by which those cells are made—and determining whether that’s important for the cell function are some of the questions that a lot of people are looking at now, and they are questions that need to be asked for every potential application,” she says.
Finding Faster Routes
A different trend Shepard has noted is an endaround maneuver some researchers are taking. “In thinking about potential therapies, people are realizing that there are a lot of hurdles to get through the regulatory process, which can be daunting given the lack of precedents for of many of these newer, cutting-edge approaches. They are looking for ways to use stem cells that might have a more straightforward path to the clinic. As a result, we’re seeing a lot of people who are trying to make products from stem cells to use as therapies.” That includes factors that are secreted by stem cells, such as exosomes (communications vesicles). By using cell products rather than the living cell itself, Shepard explains, these researchers hope to exploit unique attributes of stem cells while avoiding some of the complexities associated with cell transplantation.
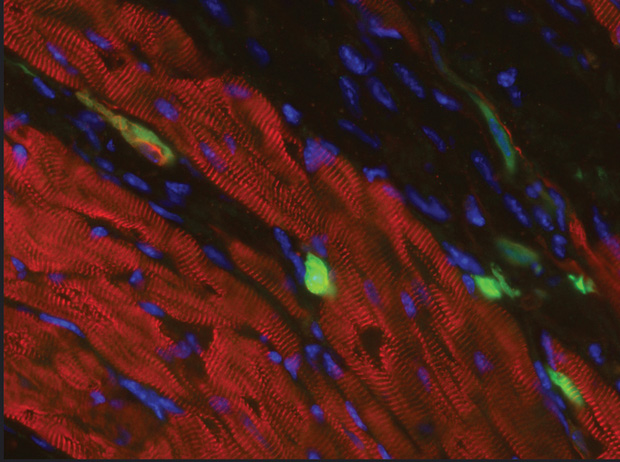
A shortcut of a different type has researchers taking the knowledge they gain from stem cells to get the results they want more quickly and easily. “For example, say you learn from stem cells which transcription factors are important for a cell to differentiate into a cardiomyocyte,” she says. “If you can express those factors in another type of cell, it might be possible to use a process called in vivo reprogramming to skip the stem-cell stage and go directly to the cardiomyocyte.” An application might be for a cardiac patient who has decreased heart function due to scar tissue (Figure 3). “If you can express key genes in that scar and convert the scar cells into heart muscle cells, that might be a completely different approach to regenerating the heart tissue that doesn’t involve actually putting cells into somebody,” Shepard says.
Beyond these individual tactics that some research groups are exploring, CIRM is also looking for more expansive ways of promoting stem-cell advances. These include pushing the application side. “We’re really looking to move things from discovery to the clinic. CIRM has laid the foundation by establishing a good understanding of mechanistic biology and how stem cells work and is now taking the knowledge and applying it for the benefit of patients,” Fitzgerald says. CIRM’s goal is to initiate 50 clinical trials by 2020. “They might be for a cell therapy, a small molecule or a biologic that’s acting on stem cells, a medical device or other tool that’s used in the delivery of stem cells, or an assay for identifying a patient population or for tracking cells once they’re transplanted.”
To that end, Fitzgerald, Shepard, and other CIRM staff are working with basic researchers to help their projects proceed down the road toward regulatory compliance, clinical trials, U.S. Food and Drug Administration (FDA) approval, and eventually the clinical market. Depending on the researcher, that help might be simple guidance, assistance in forming collaborations, or more intense support. In addition, CIRM and other stem-cell initiatives across the country are actively participating in discussions with the FDA to develop a regulatory path for stem-cell therapies that goes beyond its current approval process, which is geared toward traditional drugs and therapeutics based on small molecules, Fitzgerald explains. “This is important because we are really at the precipice of a large number—hopefully—of stem-cell therapies going into clinical trials.”
Moving Forward
It’s impossible to put a finger on one major discovery or technology that is accelerating the stem-cell field. “We’re really seeing an intersection of many things happening at once,” says Fitzgerald. That includes the emergence of big data, which is helping make sense of all the information emerging as new technologies, including whole genome sequencing, reveal the details of living things. It also includes a publicly available, CIRM-funded stem-cell bank, which began offering its first 300 stem-cell lines—covering 11 common diseases and disorders ranging from heart, lung, and Alzheimer’s disease to autism and cerebral palsy—to researchers in September 2015. The still-growing stem-cell collection is stored and distributed by the Coriell Institute of Medical Research from a facility in California. Ultimately, according to Fitzgerald, the bank will house up to 3,000 cell lines, including the genomics sequencing data for each line, so that researchers can select the lines that are most relevant to their work. Adds Shepard, “Between technological breakthroughs and discoveries, there are so many really exciting things going on and so many directions one can pursue. Now, we just need to focus and answer the questions that need to be addressed so we can push stem-cell research toward the patient as quickly as possible.”
References
- National Institutes of Health. (2009, Mar. 10). Human embryonic stem cell policy under former President Bush (2001, Aug. 9–2009, Mar. 9). [Online].
- CIRM. (2015). California: The leader in stem cell research. [Online].
- M. Jinek, K. Chylinski, I. Fonfara, M. Hauer, J. A. Doudna, and E. Charpentier, “A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity,” Science, vol. 337, no. 6096, pp. 816–821, Aug. 2012.



