Within a decade, life will likely become a lot easier for people with low back pain. The reason is cell therapy. Research is progressing rapidly and clinical trials are ongoing for new products that promise to repair the damage at the root of back pain.
These cell-therapy products are not in the same class as the bevy of currently offered but unapproved “point-of-care” treatments that use stem cells [1], according to researcher Farshid Guilak, Ph.D., professor in the Department of Orthopedic Surgery at Washington University, St. Louis, MO, and co-director of its Center of Regenerative Medicine. “There are close to 600 clinics in the U.S. that offer what they call ‘stem-cell therapies’ for thing like Parkinson’s, arthritis, and low back pain particularly, but none of those procedures have been shown in a controlled randomized study to work or are actually approved by the U.S. Food and Drug Administration (FDA),” he asserted. “That’s been very bad for the field because there are side effects from these uncontrolled and unproven therapies.”
The best way to change that impression is to develop cell-therapy products that have gone through rigorous government regulatory processes to verify safety and efficacy, and get them into the clinic so patients can see for themselves that they work against chronic low back pain. Guilak’s group is one of numerous research programs at academic labs and research companies doing just that, and are speeding forward on this new chapter for cell therapy.
Hips and More

Guilak’s group is putting much of its attention on hip pain, and has succeeded in using stem cells to regrow the entire head of a hip joint (Figure 1). He hopes to get the hip replacement into phase one human trials by about 2022.
To reconstruct the hip joint, he and his group begin by harvesting a small amount of stem cells (2) from a patient’s bone marrow or subcutaneous fat. They bathe the stem cells in media containing nutrients as well as growth factors, such as transforming growth factor beta and bone morphogenetic protein (BMP), to encourage them to develop into cartilage cells.
The trick was to turn the mass of cells into something with the form and function of a hip joint. “What we came up with is a method for three-dimensional weaving where we make a fabric from hundreds of biomaterial fibers—we use resorbable sutures that melt away over time—to create a layered material that’s flexible like cartilage, but is really tough and very strong, and we form it into a scaffold with the shape of the ball of a hip joint,” he said (Figure 2). “The scaffold has pores in it where the cells can go inside, set up shop, and then grow and form the tissues that we want, whether that’s cartilage or bone” (3).
When the scaffold eventually dissolves completely, the patient has a replacement joint made from strong, living tissue. The spin-off company Cytex Therapeutics of Durham, NC, was formed in 2006 to do the translational work, and “is at the point now of testing them in large animals, and so far they look like they’re working really well,” Guilak said.
As that work moves forward, Guilak’s lab at Washington University is also affiliated with Shriners Hospitals for Children in St. Louis (where he also holds the position of director of research) to develop arthritis protection for the newly replaced joint, so it doesn’t succumb to the same fate as the original joint. To that end, they used the gene-editing method CRISPR to engineer the new cells, adding a gene for a natural anti-inflammatory drug as well as the ability to sense the inflammation (4). “So they automatically make the drug whenever there’s a flareup of inflammation,” he said.
Tests of the drug-producing cells show that they respond very quickly to the inflammation of osteoarthritis, and turn on and off as they should, he said. The group is now developing a similar tactic for rheumatoid arthritis and looking into its application in other conditions, he said. “It’s something really exciting, and we hope it can provide a completely new use for stem cells.”
Disc Repair: BRTX-100
Other groups are putting their focus on degenerative disc disease, a common condition that can lead to near-constant and debilitating pain (5), and specifically on using cell therapy to rebuild the intervertebral disc material (nucleus pulposus) that serves as a shock absorber between the individual vertebrae.

One of the companies with significant research in this area is BioRestorative Therapies of Melville, NY, which is growing and expanding stem cells from the patient’s bone marrow, but doing so under hypoxic conditions that mimic those in the normal intervertebral space (6). “So basically we are enriching the cells to be able to survive in this harsh environment,” said Francisco Silva, the company’s chief scientist and vice president of research and development (Figure 3). The researchers combine the cells with platelet lysate, a blood component that is rich in growth factors and nutrients for the cells, and cryopreserve the final cell product, which they call BRTX-100.
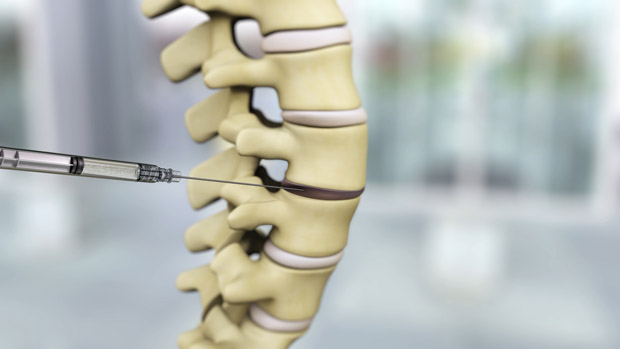
“Once the FDA authorizes the sale of BRTX-100, we would ship it to your doctor, and with a 30-minute procedure the material would be injected into your disc in a 1.5 ml solution, and that’s it,” Silva described. The stem cells function in a couple of different ways. “Stem cells by nature are anti-inflammatory (and) release factors to block the inflammation process,” he explained. The company’s initial animal study showed increased disc height following treatment (Figure 4). Another animal study is planned to examine BRTX-100’s mechanism of action, and to measure changes in pain level following treatment.
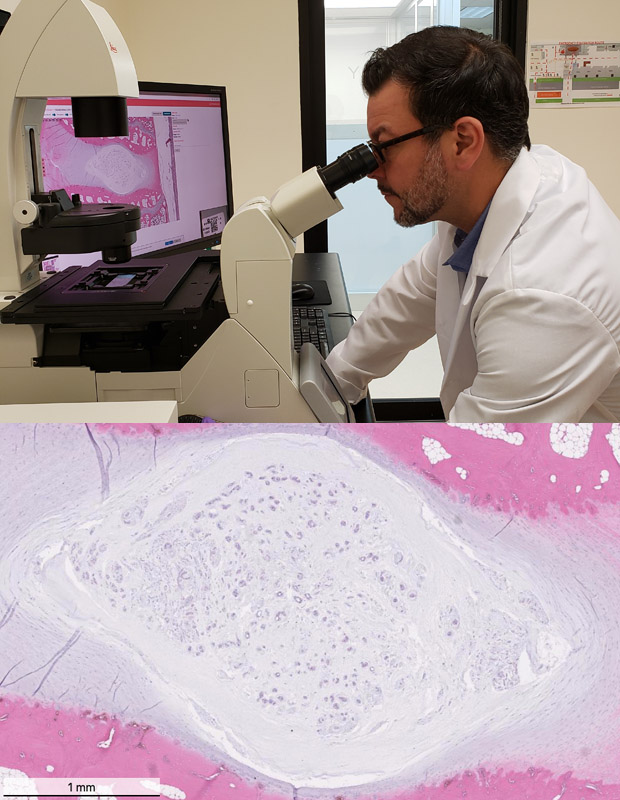
The FDA authorized BioRestorative Therapies to initiate a phase 2 clinical trial of the BRTX-100 cell product, which will provide for the study of 99 patients.
Besides the BRTX-100 cell product, the company is also pursuing an additional cell-therapy project: utilizing brown adipose (fat) derived stem cells for therapeutic purposes, according to Silva. Since brown adipose tissue expends energy (as opposed to white adipose tissue, which stores it), the company’s goal is to develop an implantable brown adipose tissue construct, which it hopes to use for the treatment of metabolic diseases and disorders, such as type 2 diabetes, obesity, and hypertension. The company is working in collaboration with the University of Pennsylvania to research brown adipose biology and its role in metabolic disorders and has also retained the University of Utah to provide research services for this program.
Disc Repair: IDCT

Another cell therapy for degenerative disc disease is also moving forward quickly. This product, called IDCT (an acronym for Injectable Disc Cell Therapy) and developed by DiscGenics Inc. of Salt Lake City, UT, has already secured investigational new drug (IND) approval and even received a Fast Track designation from the FDA in August 2019 (Figure 5). A randomized, double-blinded study of 60 subjects at 14 U.S. centers is currently under way to support an eventual Biologics License Application (BLA). The company is concurrently conducting a trial in Japan in hopes of receiving a Marketing Authorization from the Pharmaceuticals and Medical Devices Agency (PMDA) there, according to Bob Wynalek, the company’s chief operating officer.
For IDCT, DiscGenics starts with disc-derived cells, converting them into therapeutic progenitor cells—the company calls them “discogenic cells” (Figure 6)—that produce building-block molecules of proteoglycan and collagen to rebuild the extracellular matrix of the intervertebral disc, explained Lara Ionescu Silverman, Ph.D., senior director of research and development at DiscGenics.
Besides the cells’ role in re-forming extracellular matrix, which the company demonstrated in animal testing [7], the IDCT cells are anti-inflammatory and “may be able to directly modulate the pain sensation that the patients experience,” Silverman reported. She noted that the clinical trial will capture self-reported outcomes associated with pain and regeneration, as well as behavior-based outcomes. It will also include imaging to monitor progress.
While the trial moves forward, the company has turned its attention to the commercialization side, and in August, moved into a new facility where clean rooms will be built with specialized equipment to transition from small-batch to largebatch manufacturing. “This really is a young field in many ways, and so we’re trying to learn from other fields, such as the bioprocessing industry, to identify best practices and better understand how we can apply them to cell therapy manufacturing. For example, we’re doing design-of-experiment characterization, which is something classically done in biologics, but that gives us a lot of information about the robustness of our process,” Silverman said.
“We’re experiencing a big shift in the identity of our business as we grow beyond our preclinical and clinical roots and begin to build upon our existing processes and internal expertise to make sure that we’re ready for commercialization when we do reach the market,” Silverman noted. The company is also working closely with the FDA and PMDA “to ensure we’re effectively leveraging their expedited regulatory pathways, where appropriate, with the goal of getting this potentially life-changing product to patients as quickly as possible.”
In the best-case scenario and with accelerated approval from the FDA, Wynalek speculated that IDCT could make it to market in late 2022. It’s not a moment too soon, he added. “I think this is something that can help a lot of people who are disabled right now. And it’s not just middle-aged people and seniors; it’s a lot of younger people who are also suffering from this due to genetic predisposition or injury,” he said, remarking that a quarter of the population is dealing with debilitating pain from degenerative disc disease. “Our goal is to introduce a product to the market that is going to help millions of people,” he said. “We want people to know that there’s something coming.”
In the best-case scenario and with accelerated approval from the FDA, Wynalek speculated that IDCT could make it to market in late 2022. It’s not a moment too soon, he added. “I think this is something that can help a lot of people who are disabled right now. And it’s not just middle-aged people and seniors; it’s a lot of younger people who are also suffering from this due to genetic predisposition or injury,” he said, remarking that a quarter of the population is dealing with debilitating pain from degenerative disc disease. “Our goal is to introduce a product to the market that is going to help millions of people,” he said. “We want people to know that there’s something coming.”
References
- U.S. Food and Drug Administration, “FDA sends warning to company for marketing dangerous unapproved stem cell products that put patients at risk and puts other stem cell firms, providers on notice,” news release, 20 December, 2018, available online at: https://www.fda.gov/news-events/press-announcements/fda-sends-warning-company-marketing-dangerous-unapproved-stem-cell-products-put-patients-risk-and.
- Guilak, F., Awad, H. A., Fermor, B., Leddy, H. A., and Gimble, J. M. “Adipose-derived adult stem cells for cartilage tissue engineering,” Biorheology, vol. 41 nos. 3-4, pp. 389-99, 2004.
- Moutos, F. T., Glass, K. A., Compton, S. A., Ross, A. K., Gersbach, C. A., Guilak, F., and Estes, B. T. “Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing,” Proceedings of the National Academy of Sciences, vol. 113, no. 31, pp. E4513-22, 2 August, 2016
- Guilak, F., Pferdehirt, L., Ross, A. K., Choi, Y-R, Collins, K. H., Nims, R. J., Katz, D. B., Klimak, M., Tabbaa, S., and Pham, C. T. N. “Designer Stem Cells: Genome Engineering and the Next Generation of Cell-Based Therapies,” Journal of Orthopaedic Research, vol. 37, no. 6, pp. 1287-1293, June 2019.
- Arthritis Foundation, “Degenerative disc disease,” available online, accessed 23 September 2019.
- Elabd, C., Ichim, T. E., Miller, K., Anneling A., Grinstein, V., Vargas, V., and Silva, F. J. “Comparing atmospheric and hypoxic cultured mesenchymal stem cell transcriptome: implication for stem cell therapies targeting intervertebral discs,” Journal of Translational Medicine, vol. 16, published online (doi:10.1186/s12967-018-1601-9), 10 August, 2018.
- Silverman, L. I., Dulatova, G., Tandeski, T, Erickson, I. E., Lundell, B., Toplon, D., Wolff, T., Howard, A., Chintalacharuvu, S., and Foley, K. T. “In vitro and in vivo evaluation of discogenic cells, an investigational cell therapy for disc degeneration,” Spine Journal, article in press, available online, accessed 24 September, 2019.