Why are nanomaterials such a hot ticket? It’s all about size. Thanks to their small dimensions (at least one dimension fewer than about 100 nm) and, therefore, high ratio of surface area to volume, nanoparticles have some very unusual and interesting chemical and physical properties. Ranging from melting point and electrical conductivity to chemical reactivity and even their color, nanoparticle properties become size-dependent, which presents opportunities to manipulate them. Added to that, biological systems work on the nanoscale, so nanoparticles can interact with cells and cellular processes in ways that larger-scale materials can’t.
[accordion title=”Safe or Not?”]
A simple question underlies the potential of biomedical nanomaterials: Are they safe?
The answer, however, is not necessarily simple because nanomaterials come in so many different types and are used in such diverse applications, according to Steve Hankin, Ph.D., head of SAFENANO in Edinburgh, Scotland. Established in 2006 as a Centre of Excellence on nanosafety and part of the U.K.’s Institute of Occupational Medicine, SAFENANO provides industry, academia, and governments with independent, authoritative expertise and state-of-the-art facilities to enable effective risk management with nanotechnology.
“From a biological perspective, there are wide-ranging interests in using these materials in medicine for therapeutic and diagnostic applications, as antimicrobials, as food additives, and in cosmetics, to name but a few,” Hankin said. That variety, combined with the number of nanomaterials currently under extensive study, including carbon nanotubes, fullerenes, graphene, and other carbon-based nanomaterials, as well as metals and their oxides, such as gold, silver, titanium dioxide, and zinc oxide, has given individual materials-safety studies a somewhat narrow focus. “Research into nanomaterials safety is frequently performed on a case-by-case basis and with a particular research question in mind—using particular materials, cell types, and assays suited to the research question—and this does not necessarily provide a comprehensive assessment of potential toxicity.”
Bit by bit through these studies, the research pool is deepening and beginning to reveal which nanoengineered biomaterials are toxic and which are benign at different dosages and in different uses. For example, some nanomaterials are safe at low and moderate doses for their intended purposes. That, however, doesn’t mean they are completely nontoxic, said Krishnendu “Krish” Roy, Ph.D., professor of biomedical engineering in the joint department at Georgia Tech and Emory universities. “All materials have interaction with cells and tissues and are likely to have adverse effects, although those effects may be undetectable when the dose is low. The more you give, however, the bigger the effect is.” And as the dose increases, the likelihood of global systemic toxicity rises.
Roy’s research group is currently investigating those interactions to add to the general knowledge about nanomaterial efficacy and safety. “As a whole, we in the field are fairly advanced in the nanomaterials that we can make, including the different types of materials we can use to make them, but where we probably need more understanding is how different cells and different transport systems in the body are affected by these nanomaterials,” he said. “Consider a particle that is injected or inhaled: How does it transport inside the body? Which cells does it interact with? What is the sequence of that interaction not only from a therapeutic or diagnostic point of view, but also from toxicity and immune-response point of view?” Those findings vary from one system to another, from material to material or from cell to cell, and from one route of administration to the next, he said.
The shape of a nanoparticle also makes a difference. In the fall of 2013, his research group published a study demonstrating that nanoparticle shape has a profound effect on how cells internalize the particles [1]. “We wanted to look at shape, because most of the work, especially in the therapeutic or diagnostic world, has focused on spherical or near-spherical nanoparticles,” Roy said. “What we found is that the geometry of the particle not only affects the efficiency of the uptake of the particle, but also the biological mechanisms that a cell uses to internalize the nanomaterials,” he said.
In particular, Roy’s group discovered that under typical culture conditions, mammalian cells are more welcoming to nanoparticles shaped liked discs than those shaped like rods and take up larger discs and rods more efficiently than smaller ones, something that doesn’t occur with sphere-shaped nanoparticles. They also found that nanoparticle size has an effect on the specific mechanisms different cells use to draw in the particles. These findings have numerous implications. In drug delivery, for instance, it could potentially lead to a reduction in the dosage required to produce a wanted result, which could in turn reduce toxicity. He remarked, “Whether it involves toxicity, efficiency of cell uptake, or delivery to tumors or to specific organs, we believe that there will be an influence of particle shape in all of these processes.”
Particle shape, or morphology, is also something that is being considered in European regulation, following work done by Hankin and colleagues at SAFENANO to inform the European Commission on how to handle nanomaterials under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation.
As work like this continues to unravel the fundamentals about cells’ responses to nanomaterials, SAFENANO is providing a free-to-access information service [2] that focuses on interpreting and disseminating the emerging scientific evidence about the potential risks to health, as well as to the environment, arising from the development and use of nanotechnology.
The information service is an important tool but is just one aspect of the overall question of nanomaterials’ safety, Hankin said. “Decisions about the significance of risk are influenced by the degree of uncertainty. The problem of defining criteria for ‘acceptable’ and ‘unacceptable’ risks is especially important in relation to human health and the environment in the absence of safe exposure thresholds,” he remarked, noting that both scientific knowledge and an appreciation of the limits of that knowledge are both necessary to determine risks. “It requires a good understanding of the context of the risk, and it requires willingness by companies and regulatory agencies where appropriate to deal openly with these difficult, variable, and value-laden issues,” he said. “This is perhaps the most emotive and one of the most challenging aspects of risk management, which continues to evolve with influencing societal and political factors, especially in the rapidly developing area of nanomedicine.”
Fortunately, extensive programs of collaborative research are under way in many countries, and along with the international Organisation for Economic Cooperation and Development, the international Organisation for Standardization (ISO), and various governments, they are together investigating such issues as regulatory assessment, potential hazards and workplace risks, exposure routes, and effectiveness of risk-management approaches. Governments, academia, and industry are all contributing “to the safety of nanotechnology across the full product life cycle: from production through use and to disposal and recycling,” Hankin said.
He added, “There is increasing consensus that for nanotechnology to reach its maximum potential in any sector, including those seeking to use nanoengineered biomaterials, we must work not only to understand the hazards and exposure routes to minimize the risks, but also to employ responsible and reasonable protective measures whilst there is still uncertainty.”
References
- R. Agarwal, V. Singh, P. Jurney, L. Shi, S. V. Sreenivasan, and K. Roy. (2013, Oct. 7). Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proc. Natl. Acad. Sci., early edition. [Online].
- SAFENANO Knowledge Base. [Online]. Available: http://www.safenano.org/knowledgebase.aspx
[/accordion]
Researchers are now taking advantage of these characteristics to begin developing new technologies that have implications for medical care, and policymakers are looking at the safety of nanomaterials for medical uses (see “Safe or Not?”). The enormous number of research projects under way and multiple potential biomedical applications, nanoengineered biomaterials are turning the heads of researchers and clinicians around the world, and that won’t change anytime soon, according to Ali Khademhosseini, Ph.D., associate professor at the Harvard–Massachusetts Institute of Technology (MIT) Division of Health Sciences and Technology, and Harvard Medical School. “There definitely will be uses for nanotechnology, and there are already companies conducting human clinical trials on nanoparticles and drug delivery, as well as applications in imaging using things like iron nanoparticles.” (See “To Market, to Market” and “Drug-Delivery Nanotechnology Moves from Bench to Bedside.”)
[accordion title=”To Market, to Market”]
Translating Nanoresearch into Practical Products
More and more biomedical researchers and engineers seek not only to learn new things about nanomaterials but also to put that knowledge to work in the clinic.
An example is Helen Lu, professor of biomedical engineering at Columbia University in New York City, who is developing a technology that she hopes will soon make a difference in the patients’ lives (Figure S1). Her focus is on engineering complex tissues, and she is especially interested in treating damage to the anterior cruciate ligament (ACL), the main ligament that stabilizes the knee. She noted that ACL injuries annually affect some 300,000 people in the United States alone—often young women.

“The body of our work is focused on generating multiple, or in the case of the ACL, three different types of tissues simultaneously,” she said, noting that ACL in its functional state consists of five tissue regions, which enables it to connect bone to bone, specifically femur to tibia. Previous tissue-engineering approaches have focused largely on regenerating the ligament proper, while its seamless integration with bone—an integral component of its function—has been understudied, Lu said.
“To make this translatable clinically, we had to design a novel scaffold system that coaxes stem cells to grow all the tissues, which will integrate naturally into a nanoscale structure with nanoscale features,” she explained (Figure S2). The system includes a different scaffold for the ligament and for the bone. “For the ligament part, we change the architecture and nanofiber diameter, and for the bone part, we change the mineral composition,” she said. The interface has been a bit more challenging, but early studies suggest that the tissue will grow with mechanical stimulation and addition of key bioactive factors. She explained, “The hallmark of this approach lies in that while the three tissues (ligament, cartilage, and bone) formed are compositionally distinct, they are structurally contiguous and the multitissue unit is able to withstand physiological loading.”
![FIGURE S2 Helen Lu and her research group are working to understand which nanoscale parameters are relevant to stem cell differentiation. As part of that effort, they are studying whether nanofiber diameter, composition, and alignment play a role. The top images show (a) aligned and (b) unaligned fabricated polymer nanofibers, and the bottom pair show human mesenchymal stem cells cultured on (c) aligned and (d) unaligned nanofibers. [(a) and (b) courtesy of Nancy Lee. (c) and (d) courtesy of Siddarth Subramony.]](https://www.embs.org/wp-content/uploads/2014/03/35009.png)
In vitro and animal studies are progressing on the ACL work, and additional studies are under way for tissue-engineering work on rotator cuff injuries. To proceed toward the clinic, Lu is giving careful consideration to the needs of the end users. “We often think the patient is the end user, but the people who are going to implement much of these nanotechnologies are the surgeons. To that end, we have designed our system to be compatible with existing surgical procedures, and therefore to have minimal disturbance to current practice. If we don’t do that, they’re not going to use it, no matter how cool the system is.” She added, “As developers, feedback from the actual end users is extremely valuable.”
Another major consideration for her work, and for the translation of all nanotechnology work, is scalability. She remarked, “One of the challenges of translation is whether we have in our medical device industry the ability to engineer nanomaterials with high fidelity and to clinically relevant levels. The big question is: Can we have product on the shelf?”
Phillip Messersmith of Northwestern University agreed with that sentiment. “If you have the ideal particle in terms of efficacy, but it can’t be manufactured at scale and at a reasonable cost, it makes it extremely difficult to ever get that work translated,” he said. “Making a couple of milligrams of a nanoparticle in a lab may yield a dozen research papers, but for translation, the issue is whether you can manufacture those particles with precise control over composition, particle size, and particle shape on a kilogram scale and in an economical way so that a company can manufacture it and commercialize it. I think it remains to be seen how successful we will be.”
Researchers Omid Farokhzad of Harvard and Robert Langer of MIT have made some important contributions in that regard. They are designing block copolymers that can self-assemble into specific, targeted nanoparticles in a single step, Farokhzad said. This has two benefits. “First, we can create libraries of nanoparticles that very narrowly differ from each other with regard to biological, chemical, and physical properties,” he said. This offers the ability to screen nanoparticles much like medicinal chemists are able to pore through libraries of compounds to find new drug candidates. Second, the technology offers a robust, reproducible way to make nanoparticles with very specific characteristics.
“In order to translate nanoparticle technology from concept to the clinic, we have to be able to decipher the complexity of these things so we can learn what works under certain conditions,” Farokhzad said. “Then we have to be able to reproducibly manufacture them using standard unit operations to avoid unnecessary complexity.”
For nanotechnology to have a broad impact on medicine, Farokhzad encourages his researchers to look at the big picture. “We do a lot of work on understanding basic fundamentals, but the litmus test we use for how much in terms of resources and investment we put toward a particular project is reaching a conviction that what we’re about to do, if we do it well, could potentially impact human lives,” he said.
That philosophy has worked well for Farokhzad, who has 125 patents and patent applications and three biotech companies that he and Langer that have grown from their projects over the years. Langer has more than 1,000 patents and patent applications and more than 25 companies to his name. Farokhzad said, “At a high level, we’re trying to create a more targeted, more personalized medicine using nanotechnology and doing that from a variety of angles, including developing targeted nanomaterials that can bring therapeutics to a particular part of the body, or technologies that can be more effective for RNA interference including approaches to deliver RNA and other macromolecules orally, and approaches for developing combination therapies which can synergistically hit multiple targets important in cancer and other diseases.”
Farokhzad added, “We believe that it takes just as much time and just as much capital to deal with big problems as it does to deal with small ones, so we try to make sure we are tackling big enough problems. If we do and if we are successful at it, the translational part of it invariably follows, whether you want it or not. It’s just part of the process.”
[/accordion]
John Rogers, Ph.D., professor of materials science and engineering at the University of Illinois at Urbana-Champaign (UIUC), and director of the UIUC Materials Research Laboratory, added, “There are things that we will be able to achieve with these kinds of technologies that people who are out in the field and in hospitals today can’t even fathom.” For now, though, researchers have their hands full. “In my lab and in many others, we’re completely swamped by requests to address things that medical and biomedical people want and need today. I think that application pull is a very good sign that we are headed in the right direction.”
[accordion title=”Drug-Delivery Nanotechnology Moves from Bench to Bedside”]
Mucus may not be the subject of polite dinner conversation, but it is a subject that lights up the eyes of Justin Hanes, Ph.D., of Johns Hopkins University in Baltimore. He is director of the Center for Nanomedicine and the Lewis J. Ort Professor of Ophthalmology with joint appointments in several departments, including chemical and biomolecular engineering as well as biomedical engineering (Figure S3). Mucus is important because many diseases occur at mucosal surfaces of the body, including those on the surface of the eye and lining the respiratory and gastrointestinal tracts, among others.

Hanes focuses much of his attention on finding ways to get drugs past the body’s mucus and to the diseased tissues, where they can have maximal effect—and he’s beginning to win the battle. Based on his research group’s work, Hanes has started a company that already has several clinical trials planned for 2014 on drug-delivery products.
Mucus presents significant problems for drug delivery. First, it has small pores and a highly adhesive mesh to keep out bacteria and viruses as well as the typical nanoparticles under consideration for drug delivery. If the drugs can’t get through the mucosal layer, they can’t reach the diseased tissue at the interface below. Second, objects that are trapped in the mucus mesh are rapidly shed, a process that can happen as frequently as every few seconds on the surface of the eye. The surface mucus layer and the stuck objects in it are whisked away to eventually be eliminated from the body. Hanes’s research group develops technologies to help drugs skirt these defenses and reach the diseased tissue.
To overcome the pore size of the mucus, they had to find something that was just the right size. Most drugs are so-called small molecules, extremely miniscule molecules that can usually pass through the mucosal pores. The issue is that they’re too small. While they can often penetrate the mucosal surface, they also easily continue through the diseased tissue, Hanes said. “Their effects are very short-lived, because they just keep on going. It’s kind of like a fly-by.” He and his lab worked on technology to develop perfectly sized nanoparticles, each of which can carry tens to hundreds of thousands of small molecules. The nanoparticles are small enough to rapidly penetrate deep into the mucus layers, but too big to continue on through the diseased tissue (Figure S4.)
![FIGURE S4 Hanes’s research group has developed nanoparticles that can get drugs past the body’s protective mucus layer (blue) and to underlying diseased tissues (pink), where they can have maximal effect. This image contrasts the ability of drug-carrying gels and conventional particles with his group’s mucus-penetrating particles to diffuse through the mucus layer in vaginal tissue. (Image used with permission from [1].)](https://www.embs.org/wp-content/uploads/2014/03/35076.png)
For the problem of stickiness, Hanes’s research group turned to an unlikely coating. “The molecules we most often use for the coating are based on polyethylene glycol, or PEG, which is widely used in pharmaceutical products,” he said. “When we first considered working with PEG, we learned it was thought to be adhesive to mucus, but it turns out that’s only the case at certain molecular weights. Higher molecular weights of this molecule appear to be more adhesive. Of course, PEG of any molecular weight is not useful unless the density of the molecule on the nanoparticle surface is extremely high.” A very dense surface coating of low-molecular-weight PEG did the job.
The resulting drug-delivery nanoparticle “penetrates the protective mucus layers and sits at the interface with the diseased tissue, where it slowly biodegrades and releases its drug,” Hanes described. “There are a huge number of diseases that occur at mucosal surfaces where therapies have been suboptimal for a long time. This new technology allows us to start to address that.”
Called mucus-penetrating particles, or MPPs, this technology is now being commercialized through the start-up company Kala Pharmaceuticals, launched in 2009 and located in Waltham, Massachusetts. “We have applied this technology to all sorts of different things,” Hanes said. “For instance, in the female reproductive tract, we showed that if we deliver a microbicide drug—an antiviral drug—for herpes simplex virus into the vagina of mice and then try to infect them with herpes virus, the drug is basically ineffective. Only at a very high dose do we start to see some effect. But if we take that same drug, make it into an MPP, and deliver it into the vagina, we can use ten times less (of the drug) and get a much better effect” (Figure S5) [1].
![FIGURE S5 In vivo images illustrate the superior diffusion of MPPs over CPs. The images contrast the particle distribution of red fluorescent nonbiodegradable (non-BD) and biodegradable (BD) CP and MPP in transverse cryosections of estrus phase and induced estrous (IE) phase mouse vaginal tissue. (Image used with permission from [S1].)](https://www.embs.org/wp-content/uploads/2014/03/35084.png)
Kala Pharmaceuticals is also investigating ways to deliver drugs more effectively to various organs, with an emphasis on the eye. At Kala Pharmaceuticals, the researchers are developing topically applied products that rapidly coat and penetrate the mucosal tissues and treat severe ophthalmic diseases, such as dry eye disease. It has four clinical trials on the docket for 2014.
Hanes is also interested in the potential for the MPP technology in other areas. “We’re using it to develop vaccines that are more effective when administered to mucosal surfaces. Most pathogens interact initially with your immune system at mucosal surfaces, and it’s known that you can get better protection when you immunize at those entry points to the body,” Hanes said. With funding from the Bill & Melinda Gates Foundation, for instance, he and his research group are exploring the use of MPP for tuberculosis vaccines.
In addition, Hanes launched a second company called GrayBug in 2011. Located in Baltimore, Maryland, the company’s focus is on injectable drug-delivery systems directed against neovascular diseases, those that involve the growth of new blood vessels. “Much like there’s a blood–brain barrier, there’s a blood–ocular barrier,” Hanes said. These barriers can keep drugs out. “There’s a lot of blinding eye diseases and other problems associated with the eye, and while we have drugs that can work to help many of these conditions, often the only way to get them into the eye is to inject them. When you do that, many of these molecules are cleared so rapidly that they don’t last, and people can’t come in every day or even every week for an injection into their eye. You need something that lasts months.”
GrayBug’s technology is designed to do just that, and it is being applied to treat important diseases that cause blindness such as age-related macular degeneration (AMD) and glaucoma. GrayBug’s approach to AMD is to create drugs that last four to six months and that stop the growth of new blood vessels that leak, leading to blindness, and cause these defective blood vessels to melt away. Hanes can’t divulge too many details at this time but noted that GrayBug has “a couple of platform technologies” that allow protein drugs and small molecules to be injected into the eye as infrequently as twice a year to produce the wanted medical outcomes. “At a high level, I can say that both platforms appear to make polymers that are commonly used for drug delivery safer for use in the eye,” he said. In addition, one of the polymers—a unique one for which GrayBug has the rights—”gives much better continuous drug release profiles; appears safe; is compatible with different drug molecules, small molecules, proteins, peptides, and other biologics in general; and has some significant advantages compared to traditional polymers such as poly(lactide-co-glycolide), or PLGA, that are under study for drug delivery into the eye.”
Work like this has great potential. “This can revolutionize the treatment for diseases of the eye that are caused by new vessel growth, such as age-related macular degeneration or diabetic retinopathy,” Hanes said. “If we can get the frequency of injections down to every six months, it would be a huge win. And that’s something we’re working on.”
When it comes to nanoengineered drug delivery, this work is just the tip of the iceberg. “I think it is safe to say that nanotechnology is going to have a strong impact on medical care in the future. It’s definitely going to happen,” he said. “Nanoparticles are already having an impact on cancer, and I think they will have an impact on a lot of diseases.”
He pointed to collaborative work between Johns Hopkins University and Virginia Commonwealth University on a system that only makes its gene-therapy product when it’s inside a tumor cell. “That’s pretty remarkable, because it allows the delivery of potent biologic agents while greatly decreasing side effects. Those are really important things.” Other cutting-edge research Hanes believes has potential impact includes the North Carolina company Liquidia Technologies that is making shape-controlled particles, which could be useful in the development of engineered vaccines and inhaled therapeutics; work at the University of Virginia that combines ultrasound with drug-delivery systems [3]; and research at Johns Hopkins into dendrimers and their potential to reverse the effects of cerebral palsy [4].
As more research is done, the number of applications only rises. He remarked, “With a good drug-delivery system, you can get very uniform drug-release rates and that can make drugs safer just in and of itself. If you add on to that the ability to target and the ability to get drugs into cells, or the possibilities for new therapies with small molecules, gene therapy, or other biologics, you can see that the drug-delivery field is going to play an increasingly important role in pharmaceuticals.”
Hanes added, “Nanotechnology is absolutely a key way to achieve better therapy through targeted drug delivery. It’s where all the action is right now.”
References
- L. M. Ensign, B. C. Tang, Y.-Y. Wang, T. A. Tse, T. Hoen, R. Cone, and J. Hanes, “Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus,” Sci. Transl. Med., vol. 4, no. 138, pp. 138–147, June 2012.
- H.-E. Bhang, K. L. Gabrielson, J. Laterra, P. B. Fisher, and M. G. Pomper, “Tumor-specific imaging through progression elevated gene-3 promoter-driven gene expression,” Nat. Med., vol. 17, no. 1, pp. 123–129, 2011.
- K. Timbie, C. Burke, E. Nance, G. Woodworth, G. W. Miller, J. Hanes, and R. J. Price, “Ultrasound-targeted delivery of systemically administered therapeutic nanoparticles,” J. Acoust. Soc. Am., vol. 5, no. 5, pp. 40–47, Nov. 2013.
- S. Kannan, H. Dai, R. S. Navath, B. Balakrishnan, A. Jyoti, J. Janisse, R. Romero, and R. M. Kannan, “Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model,” Sci. Transl. Med., vol. 4, no. 130, pp. 130–146, Apr. 2012.
[/accordion]
Building Scaffolds and Tissue
A rapidly expanding field within nanotechnology is tissue regeneration, an area that may one day provide an alternative to organ transplantation and other therapies to repair damaged tissues. To do it right, tissue regeneration must begin with the construction of a scaffold that mirrors the nanostructure of the body’s extracellular matrix, which provides the tissue structure upon which new cells can grow. Researchers are turning to nanocomposite hydrogels and other nanomaterials that interact well with cellular components to orchestrate the generation of fully functional tissue of different types [1].
Nanomaterials with unique properties and nanoscale features are designed and developed to mimic native tissue properties. “Synergistic interactions between nanomaterials and stem cells offered great prospects to address some of the daunting challenges in regenerative medicine,” said Akhilesh K. Gaharwar, Ph.D., assistant professor the Texas A&M University. Specifically, the interactions between stem cells and their microenvironment play key roles in controlling stem-cell fate, which underlies the therapeutic success. Most of these nanomaterials are used as scaffolds to facilitate the formation of functional tissues or to deliver therapeutic agents, he explained.
However, the interactions between nanomaterials and stem cells are still not fully understood, Gaharwar said, so there is a need to understand how nanomaterials interact with the human body. Some of the potential applications of nanomaterials in stem cell research include tissue regeneration, stem cell isolation, immunomodulation, cellular and molecular therapies, cancer research, and drug/gene delivery. “The enhanced understanding of nanomaterial-stem cell interactions will benefit us in designing advanced biomaterials for a range of biomedical and biotechnological applications,” he said.
Hydrogels have become alluring because they are so like the body’s own tissues. “A hydrogel is any system that has solidlike mechanical properties but contains a large fraction of water in its composition. A large fraction can be anywhere from 70% water, which is more in the range of human tissue, to as much as 99% water,” explained Bradley Olsen, Ph.D., a hydrogels researcher and an assistant professor of chemical engineering at MIT. “The ability to match the physical and mechanical properties of a natural biological system allows you to imagine creating a synthetic tissue or a tissue-engineering matrix. That makes hydrogels a very attractive technology.”
Another enticing characteristic of hydrogels is that their material properties can be easily tuned. “By manipulating the architecture of these materials, we can control how cells interact with them. And we can do that by integrating nanoscale materials into the gels and actually control the properties of the gels,” Khademhosseini said. “There are a lot of ways in which we can integrate nanomaterials into different types of gels and, through that, regulate cell function.”
As an example, Khademhosseini’s research group is using nanofibers and nanomaterials to replicate heart tissues. “We add nanomaterials to our gel scaffolds to help the cells align in a particular way to make the right connections with each other [2]. At the same time, the nanomaterials control material properties, so if we design it properly, the material can become conductive.” With that conductivity, these engineered tissues respond to electrical stimulation just as the native heart does, conceivably allowing them to be used for transplantation.
And because those tissues so closely mimic heart tissue, they also could be used for other purposes such as drug testing. One motivation is to move away from using animal tests, not only for ethical reasons but also because animals are not really predictive of human response. “If one can instead use cells to generate human tissues, and perhaps even link different types of human tissues to put forth a more complicated kind of organism response, then you can potentially reduce the need for animal tests,” he added.
Khademhosseini and Gaharwar are also using synthetic silicate nanoplatelets, known as layered clay and composed of salts of silicic acids, to induce stem cells to differentiate into bone or cartilage. “If we consider how bone or cartilage develops, it involves the upregulation of orthosilicic acid, magnesium, and other minerals that trigger the embryonic stem cells to become bone or cartilage. We are taking the same approach,” Gaharwar said. Their paper in Advanced Materials in June 2013 [3] showed that these nanoparticles can provide the signal that stem cells should go into an osteogenic lineage.
On the basis of this work, the researchers are collaborating with companies and clinicians to pursue development of a medical product for clinical use. They are also in the early stages of developing injectable nanocomposites that combine these nanoparticles with polymeric matrix, Gaharwar said. “The basic idea is to inject this into the site of damage or other defect. The nanoparticles will attract stem cells that will start differentiating into bone.”
Olsen’s MIT research group is developing a nanostructured injectable protein hydrogel that alters its properties with temperature change (Figure 1). “We’re very interested in understanding how to control the physical responses and properties of the gels such that they are more faithful mimics for many aspects of biological tissues,” he said.
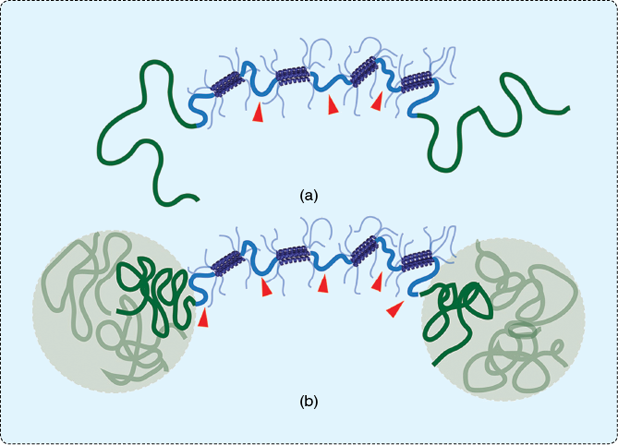
The goal was to create hydrogels that could be injected as liquids into the correct location in the body and, once there, would harden into sturdy solids. “Many researchers have been attracted to the area of making and using hydrogels with higher toughness, and there’s been a vast amount of groundbreaking work in this area,” Olsen said. “Our contribution was making gels where we could turn this toughness on and off.” They did it by grafting thermoresponsive groups onto a hydrogel, technically called an artificially engineered protein gel. The gel is then injected into the body as a rather chilly liquid and, with the increased temperature inside the body, sets up into a solid.
Olsen envisions the work having applications for tissue repair. He said, “If you have tissues that are weakened and need temporary reinforcement, this could provide a matrix for regrowth using cell-based therapies.” He’s also working with Khademhosseini, Gaharwar, and Gareth McKinley of the MIT Department of Mechanical Engineering on injectable biomaterials that can stop bleeding. “What we’d like to do is develop something that can be injected into the wound site and create clotting until that person can receive definitive care,” Olsen said. They’ve applied for a patent on the technology, which adds clot-promoting synthetic silicate nanoplatelets into a protein gel mix. It’s still in early testing, but he envisions it being used by emergency-response personnel or by medics in a war zone to help keep patients alive until they can reach a hospital.
Acute and chronic skin wounds can benefit from nanomaterials too, according to Seeram Ramakrishna, FREng, FNAE, professor at the Center for Nanofibers and Nanotechnology, National University of Singapore. He uses electrospinning, a technique that employs an electrical charge to draw nanofibers from a liquid. In this case, the liquid contains dissolved biopolymer polycaprolactone and aloe, a popular substance used in Chinese and Ayurvedic medicine to confer antimicrobial, anti-inflammatory, and wound-healing properties [4]. The resulting fibers are then constructed into scaffolds. “Nanofibrous scaffolds, with their high surface area and porosity, are desirable for high-density cell and tissue cultures,” he explained. The scaffolds act as a wound dressing, providing a structure that not only protects the wound bed from microorganism invasion but also encourages maximal growth of fibroblasts, the principal cell in connective tissue, which in turn promotes tissue regeneration, he said.
Beyond the skin, Ramakrishna is also studying nanomaterials for treating heart attacks. He is using a biocomposite of poly(L-lactic acid)-co-poly(e-caprolactone)/collagen, or PLACL/Col, to generate nanofibrous scaffolds that copy the native myocardial environment, with hopes of one day replacing some of the open-chest surgeries, transplantations, and left ventricular assist devices that make up today’s arsenal against myocardial infarction. “Surprisingly, cell morphology, growth, and expression of an interactive healthy cardiac cell population (are) exquisitely sensitive to differences in the composition of nanoscale scaffolds,” he said. This indicates that PLACL/Col scaffolds make “good prospective biomaterial for myocardial regeneration that can be potentially applied in cell-based therapies.” Overall, he added, “Electrospinning of synthetic and natural polymers enables creation of truly biomimetic scaffolds for tissue-engineering applications.”
Just Print It
Three-dimensional (3-D) printing and manufacturing also provide new avenues for nanotechnology. By integrating nanomaterials into larger 3-D structures, such as scaffolds, “we have the opportunity to offer really exquisite control over the architecture of materials,” Khademhosseini said.
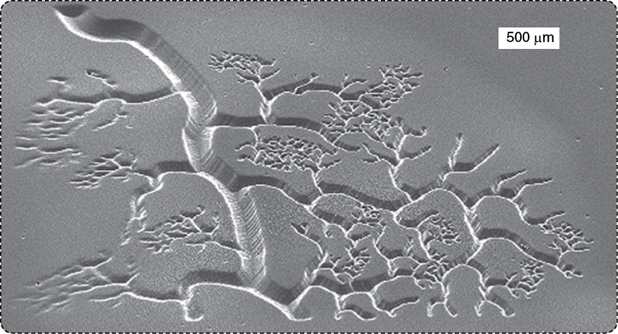
Shaochen Chen, Ph.D., nanoengineering professor at the University of California, San Diego, and his research group are doing just that. They are working on a novel 3-D printing technology that can fabricate branching blood vessels out of biocompatible hydrogels [5]. “It’s important for tissue engineering to create vasculature architectures in the scaffolds or other hydrogel materials to support the cells,” he said.
Vasculature has been challenging for a few reasons, including the multiple vessel sizes needed. “If you look at vasculature, there are larger tubes that branch out into smaller ones and eventually into very small tubes that are a couple of microns across,” Chen said. The current 3-D printing technology didn’t have the capacity to print such a wide size range, so he and his research group developed a new technology that did. “We can now print those structures ranging from a couple of microns all the way to the millimeter scale within a second.”
To do it, they exchanged the typical printer nozzle method with a new technology that uses light-sensitive polymers, or photopolymers. “We can focus light down to the nanoscale and then shine light on the photopolymers to turn them from liquid to solid.” Known as dynamic optical projection stereolithography, their system exercises computer-aided design to direct the light via micromirrors, so they not only have much-better-than-pinpoint control of the structures they build but can also construct structures with the wide size variability of a blood-vessel network (Figure 2).
Another advantage is that they can manipulate the printing medium. “With polymers in a liquid state, we can add things. We can put cells inside or put nutrition inside, and then when you shine the light, you can lock everything in that light spot,” Chen said.
That process is critical for 3-D structures, such as scaffolds. “With scaffolds, people usually use so-called pulsed cell seeding, in which you make a structure and then try to squeeze cells inside the scaffold so they grow into the shape you want for tissue engineering or organ printing,” Chen said. The trouble is cells don’t comply. “They don’t want to go inside, because there’s not that much nutrition inside. They would rather stay on the surface,” he explained. “The other other thing is that they have to discharge their waste, and that’s harder to do when they’re inside. But if you lock them up inside the scaffold, they have no other choice. They have to stay there.” The tissue can therefore grow throughout the scaffold, rather than just on the outside. The result is a living structure with cells inside that are biologically and chemically functional.
Chen and his research group are focusing on heart and liver applications. “For cardiac tissue regeneration of damaged heart tissues, the idea is to grow the cells in a scaffold that is designed as a heart patch, so it would be put on the heart surface as a way to grow replacement tissue,” he said. They are also working on regenerating liver tissue. “We’re using those biomimetic structures to simulate the native architecture of the liver, and then, by adding liver stem cells, we hope to regrow the liver and the liver functions, including removing toxins.” Animal studies are under way for both the cardiac and liver tissue projects, and they plan to conduct in vivo experiments in the future.
Bioinspired Design
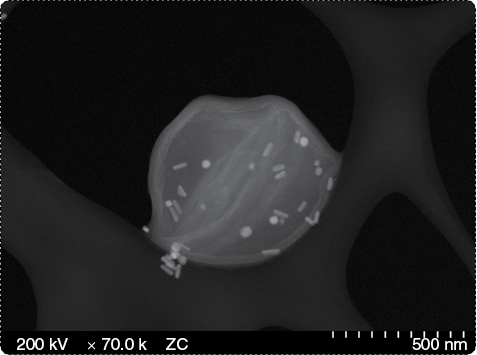
A primary focus of research into biomedical nanomaterials is to mimic various aspects of biological tissues. Some researchers are taking that to another level. One of them is Phillip B. Messersmith, Erastus O. Haven Professor of Biomedical Engineering at Northwestern University in Illinois. He is borrowing features of the specialized proteins that mussels secrete and use to attach to wet surfaces (Figure 3). “Using key features of those proteins, we are developing novel synthetic surface chemistries to attach biomolecules to surfaces of nanoparticles or to attach polymers that can both encourage very specific tissue targeting,” he said. This also inhibits the uptake of particles by nontarget cells.

Messersmith and his research group chose mussels as a model because the organisms live in a fluid not unlike that found in the human body. The environments for both are mostly water, have a high salt concentration, and have a similar pH. Beyond that, mussels have evolved very elegant adhesive chemistry for anchoring onto surfaces in water, he said. “Why not learn from organisms that have evolved over millions of years to solve those problems?”
So far, the research group has discovered many of the secrets of the mussel proteins’ surface chemistry. Some intellectual property stemming from that understanding has already been licensed, but it’s not quite ready for translation to the clinic. Envisioning future uses in diagnosing or treating cancer, he estimated anywhere from three to eight years before any technologies are implemented in a medical device or clinical therapeutic or diagnostic strategy. “The idea is to use bioinspired surface chemistry to get these particles into the body and to the tumor tissue, ideally directly in contact with or ingested by the cancer cells,” he said. The particles would begin to work—perhaps releasing drugs or yielding local heating to kill the cells—when they get the appropriate signal. The signal could come from some change within the targeted cells themselves or from an external stimulus such as light.
The technology has possibilities for seeking out and destroying bacterial cells too, and his research group is exploring that as well as coatings that are inspired by compounds found in foods and beverages, like chocolate, coffee, tea, and wine, Messersmith said (Figure 4). “It turns out that these compounds, which have health benefits, happen to have similar chemical properties to the mussels’ adhesive proteins, so we’re now studying plant-derived materials.”
Rogers’ group at UIUC also took a cue from nature in developing stretchable nanomaterials that can be printed with electronics (Figure 5). “Current integrated-circuit and silicon-device technologies work well for commercial electronic devices such as cell phones and laptops, but biological systems present a profound mismatch in mechanics and geometry between the organs of the body and today’s planar integrated circuits and semiconductor wafers,” he explained. “If you want to take that technology and bring it to bear on important problems in human health, you need to integrate those devices in an intimate way with the soft, curvilinear tissues of the human body.”
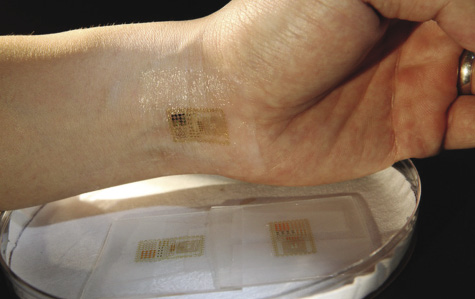
In other words, the electronic device must take inspiration from actual tissues: besides being small, it must conform to to tissues as well as flex and stretch with them.
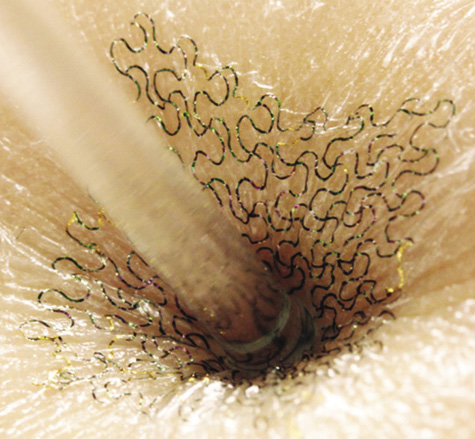
Rogers’ group tackled the problem by shaving ultrathin ribbons or membranes of silicon in the nanometer range and then printing the very fragile silicon nanoribbons and nanomembranes onto an elastomer rubber substrate. To give the printed electronics even more elasticity, they did something rather ingenious: they stretched out the substrate as they added the nanoribbons and nanomembranes. “The substrate is in a state of tension when we bond the materials to it, and when we relax that prestretch, it induces compressive stresses on the silicon, and those stresses cause the silicon to buckle into a kind of wavy geometry,” he said (Figure 6). “In that shape, we can affect end-to-end stretchability in the wavy silicon without cracking the material. So basically, the mechanics are defined by the rubber substrate, and the silicon wave structure is kind of along for the ride as you stretch it.”
While he can think of a full range of conceivable applications, Rogers really doesn’t have to: Researchers in bioengineering and in medical schools are seeing the capabilities and identifying areas where this kind of technology could be useful in their worlds. “The applications have come to us,” Rogers noted. “It’s very much an application pull rather than a technology push.”
As an illustration of that pull, the research group is currently working with others who are seeking nanotechnology-assisted solutions involving the brain, heart, and skin. For the brain, the goal is to develop electrical systems for mapping out high-resolution electrical activity on the surface during surgical procedures used for epilepsy. Animal trials have provided excellent results, and human trials may begin soon.
“For the heart, we’re working with companies and also medical school experts on devices,” Rogers said. These include devices mounted on catheter balloons that map electrical activity, blood flow, and temperature inside the heart, and use radio-frequency ablation to treat cardiac arrhythmia. Advanced animal trials are completed, and experiments have been conducted on explants from human organ donors.
For skin, wound healing is the goal. In partnership with the Northwestern University Medical School, Rogers’ team is using ultraprecision temperature and hydration sensors with skinlike forms that mount close to, and in some cases directly on top of, surgical site wounds. These sensors measure the healing process and identify any emerging infections very early on. Devices have already been used with patients at Northwestern medical centers and at a local hospital.
The research group is also beginning to investigate how the technology may be employed to generate chemical and biological sensors, with potential uses in glucose monitoring or other applications. “We think this is an appealing way to exploit nanomaterials,” he said. “The nanomaterials are providing the electronics and sensing functionalities, and the soft, elastomer matrix material is providing the kinetics and shape for effective integration with biology.”
Special Delivery
A major area of study is targeted drug delivery, in which nanoparticles are loaded with drugs, tagged with homing molecules that direct them to the disease site and into the targeted cells, and then made to slowly release the drug payload where it’s needed, when it’s needed, and at the rate at which it’s needed.
Of the seven targeted nanoparticles already in the clinic, two of these are based on work conducted by Omid Farokhzad, M.D., associate professor of anesthesia at Harvard Medical School and director of the Laboratory of Nanomedicine and Biomaterials at Brigham and Women’s Hospital, and collaborator Robert Langer, Prof. David H. Koch Institute Professor at MIT. In November 2013, the two researchers shared one of the largest nanotechnology prizes, the RUSNANOPRIZE, for the development and industrialization of nanoparticle technologies for medical applications.
The use of nanomaterials in drug delivery (see “Drug-Delivery Nanotechnology Moves from Bench to Bedside”) has numerous benefits, one of which is the prospect of giving drugs more safely and with greater efficacy, Farokhzad said.
Some researchers are exploring the idea of reducing drug dosage. “Because nanoparticles have such a high surface area, some can be designed to absorb lots of drug on their surfaces, and through particular stimuli, they can release those drugs on demand and at the targeted tissue,” Farokhzad said. Rather than the current practice of flooding the body with the drug so that at least some portion will reach the disease site, nanomedicine is so much more efficient that the dosage can be drastically lowered in many cases. With the reduced dosage, side effects plummet or disappear altogether, he explained.
The seven targeted nanoparticles in the clinic today are just the beginning, Farokhzad said. He anticipated that one or two targeted particles will be approved for clinical use in the United States in this decade, with another seven or eight nanoparticles in the following decade, and even more in the decade after that. He remarked, “The impact 20 or 30 years from now when we look back at it will be massive.”
Nano is Big
Governments have duly noted the prospects of nanomaterials in biomedicine and other fields. The U.S. federal budget for 2014 provides more than US$1.7 billion for the National Nanotechnology Initiative [6], an interagency effort to encourage nanotechnology R&D in academic, government, and industry laboratories across the country. Other countries are also making large investments. According to estimates from 2008 [7], the governments of the European Union invested about US$1.7 billion in nanotechnology; Japan invested US$950 million; China, US$430 million; Korea, US$310 million; and Taiwan US$110 million. In 2008, U.S. government spending reached about US$1.55 billion.
Nanotechnology will only increase in the coming years. A demonstration of the pending rise lies in employment statistics. In 2000, the number of nanotech workers worldwide totaled about 60,000, then jumped to about 400,000 in 2008, and it is projected to rise to 6 million by 2020 [8]. Research publications also reveal a steep upward curve. “If you checked in PubMed, there were about 310 articles published on nanoparticles in 2000. If you did the same search at the end of 2010, in the year 2010 alone 11,800 articles were published, and in 2011, 2012, and 2013, we are now at about 15,000 articles per year,” Farokhzad said. “The number of publications coming annually has exploded.”
He likened the fast rise to a similar boom that occurred in the field of monoclonal antibodies. “In 1980, there were about 500 papers published, and by 1990, there were about 10,000 papers per year. Today in 2013, some 20 years after the U.S. Food and Drug Administration’s approval of the early monoclonal antibodies such as rituximab in 1997, biologics now represent about a $35 billion market,” Farokhzad said.
He added, “If you give it the time that it needs over the next 20 so years, I think the impact of nanotechnology on medicine will probably dwarf the impact that we saw in biologics” [9].
Khademhosseini agreed that nanoengineered biomaterials are only at the beginning of a long path forward. “There’s always the hype, then there’s the reality check, and then there’s the maturing of the field. Nanotechnology is still a maturing field, but it’s clear that the opportunities are out there.”
References
- A. K. Gaharwar, N. A. Peppas, and A. Khademhosseini. (2013, Dec. 6). Nanocomposite hydrogels for biomedical applications, Biotechnol. Bioeng. [Online]. To be published.
- S. R. Shin, S. M. Jung, M. Zalabany, K. Kim, P. Zorlutuna, S.B. Kim, M. Nikkhah, M. Khabiry, M. Azize, J. Kong, K. T. Wan, T. Palacios, M. R. Dokmeci, H. Bae, X. S. Tang, and A. Khademhosseini, “Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators,” ACS Nano, vol. 7, no. 3, pp. 2369–2380, Mar. 2013.
- A. K. Gaharwar, S. M. Mihaila, A. Swami, A. Patel, S. Sant, R. L. Reis, A. P. Marques, M. E. Gomes, and A. Khademhosseini, “Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells,” Adv. Mater., vol. 25, no. 4, pp. 3329–3336, June 2013.
- J. Venugopal, R. Sridhar, R. Rajeswari, M. Shayanti, R. Balamurugan, and S. Ramakrishna, “Nanofibrous-structured biomimetic strategies for skin tissue regeneration,” Wound Rep. Reg., vol. 21, no. 1, pp. 1–16, Jan.–Feb. 2013.
- A. P. Zhang, X. Qu, P. Soman, K. C. Hribar, J. W. Lee, S. Chen, and S. He, “Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography,” Adv. Mater., vol. 24, no. 31, pp.4266–4270, Aug. 2012.
- U.S. Government. NNI Supplement to the President’s 2014 Budget. [Online].
- Nanotechnology research directions for societal needs in 2020: Retrospective and outlook. [Online].
- M. C. Roco, “The long view of nanotechnology development: The national nanotechnology initiative at 10 years,” J. Nanopart. Res., vol. 13, no. 2, pp. 427–445, Feb. 2011.
- N. Kamaly, Z. Xiao, P. M. Valencia, A. F. Radovic-Moreno, and O. C. Farokhzad, “Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation,” Chem. Soc. Rev., vol. 41, no. 7, pp. 2971–3010, Apr.2012.