Innovation isn’t easy. Not every idea is great, and not every great idea evolves into a final product or solution. So what does it take to move from the drawing board to realization, especially in the field of biomedical engineering, which seems to be churning out one great idea after another? It’s not about luck, according to the experts. The road to success has many twists and turns, but by passing a few guideposts along the way, the journey can be a rewarding one.
1. Identify an Unmet Need
“The most important thing is your starting point, and there are two different models for reaching it,” said Tony Voiers, chief executive officer of Novocor Medical Systems. “In one model, which is more traditional, you develop an interesting technology and then go looking for a home for it. I’m not a subscriber to that model anymore. Although there are a lot of successful companies that have gone that route, there are also a lot more unsuccessful ideas that never made it out because they are basically a hammer looking for a nail.”
The better model is to find an unmet need first, Voiers said. “If you can come up with a solution to that unmet need, you know you already have a market built in.” An example of that philosophy is Voiers’ company, Novocor, which emerged from an unmet need identified by undergraduate students in a 2007 capstone course offered by the joint department of biomedical engineering at the University of North Carolina (UNC) and North Carolina State University.
To learn the basics about innovation development, “the students spend time in the field looking for areas where clinicians or other medical professionals are having a problem or an issue, or where medicine could be delivered better, and then innovating around that and figuring out how to improve it,” Voiers said.
At one point, students rode in the backs of ambulances and watched the paramedics in action. One of their observations was that the paramedics needed immediate access to ice-cold saline, which is used to slow metabolism and reduces tissue damage in cardiac-arrest patients. The ambulances, however, had no refrigerators and lacked the power or space to add one. As a result, treatment was delayed until another vehicle could arrive with the cold saline, Voiers said. The undergraduates’ solution was to save both time and the cost of dispatching a second vehicle by developing small, easily stowed, instant chemical cold packs that could chill the saline as it’s being delivered, he explained. “That is exactly where the Novocor idea came from.”
The medical device design course at the Massachusetts Institute of Technology (MIT) also teaches students to look for unmet needs. Here, however, clinicians bring their problem areas to the students, said Nevan Clancy Hanumara, Ph.D., who manages and teaches the hands-on medical device design course at MIT with Prof. Alex Slocum and Prof. Charlie Sodini. Hanumara is also the resident postdoc in MIT’s Precision Engineering Research Group. “The clinicians actually compete to work with our students. They desperately want to be innovators, and the ones we work with in the Boston area are leading clinicians who want to work together to design something that will make everybody’s life easier.” In the fall 2013 semester, nine clinical projects went through a 14-week design process to result in a proof-of-concept prototype.
Striving for better patient outcomes is a major driver of biomedical innovations. This extends well beyond industrialized countries, where wealthier populations have an insatiable demand for the best new technologies. “There’s a huge population in the world that does not have access to quality health care, so we must come up with efficient solutions for them too,” said Hanumara, who is also affiliated with MIT’s Tata Center, which places one of its focuses on developing quality health care solutions for developing countries. Specifically, its fellowship program brings students’ technical education to bear on projects that benefit emerging India.
Tata Center efforts in 2012 included projects to design and fabricate a high-performance, low-cost prosthetic knee; to develop a prosthetic foot that can be mass-manufactured and produced inexpensively; and to tap into the ubiquity of cell phones and their increasing capabilities for the purpose of developing mobile-imaging platforms that address medical diagnostic and imaging needs.
Although the ideas generated from the fellows at the Tata Center are developed for emerging economies, Hanumara pointed out that they aren’t confined to them. “If you come up with an efficient solution that can provide top-quality health care in a developing economy, there’s no reason we can’t learn from that here,” he said.
2. Choose Wisely
“‘Innovation’ is an easy word to bandy about, but it’s not so easy to see which specific areas are going to respond to that in the quickest and most profitable ways,” said Sodini, LeBel Professor of Engineering and part of MIT’s Microsystems Technology Laboratory. He is also the founder and codirector of the Medical Electronic Device Realization Center (MEDRC), a collaborative effort to stimulate innovation.
Sodini is involved in one of the most rapidly expanding areas of health care innovation: microelectronics. “Microelectronics has been disrupting industries for the last 30 years: watches and calculators in the 1970s, computers in the 1980s, and communications and consumer electronics over the last two decades. Medical devices are really poised to be in that same kind of category,” he said.
In selecting the areas that had the most promise, the MEDRC settled on three: 1) developing wearable and minimally invasive monitors with diagnostic quality; 2) finding ways to lower the cost of imaging (perhaps through wearable ultrasound or other systems); and 3) point-of-care solutions that move lab analysis functions from the medical lab to the patient’s home, Sodini said. The latter would be especially beneficial for the growing number of older patients who need frequent blood work or other tests but have difficulty getting to and from the medical lab. He remarked, “The whole idea here is to use microelectronics to push these diagnostic and monitoring capabilities closer to the patient.”
Beyond microelectronics, other areas will advance health care. One of them is microfluidics. “Microfluidics has been out there for years, but other than glucose monitors, it hasn’t taken off quite yet. I think there will be a number of these kinds of monitors becoming available in the next four or five years,” Sodini said.
3. Make It Enticing
It’s not enough to come up with a great idea. That idea has to be nurtured and developed into something that will turn an investor’s head, said Ken Nisbet, associate vice president-technology transfer at the University of Michigan (U-M), one of the largest research universities in the world with 2013 research expenditures topping US$1.3 billion. “I think of the word ‘innovation’ as a combination of creativity, imagination, and looking at problems while seeking solutions,” Nisbet commented. At universities, creativity and imagination run rampant, and while they are a powerful source of new discoveries and innovations, marketable products are often an afterthought, he added.
“The major research universities are by nature focused on basic research and testing the boundaries of knowledge, often with funding from the government, so the findings are very, very early in the development process,” he said. “Innovation, however, is not just discovering something, but also applying it to a societal need. Trying to take these early discoveries and turn them into market solutions is often quite difficult,” Nisbet said.
To do this, “the research talent that conceived of that original idea needs to be complemented by other talent, such as applied-development talent and market-awareness talent. We need to find people who can help steer the project to the point where we can interest a market partner,” Nisbet said. That kind of nurturing is also necessary to attract investors, who are reluctant to put their money into such early-stage projects because of the risk involved. “At a university, we never really produce something that can be put on the market tomorrow. It has to go from us into the hands of an existing company or a start-up company that can finish the job of commercializing the new innovation.”
Companies need to step up, too, Hanumara said. On the one hand, they know that innovation is critical because having the latest and greatest product keeps the company on top. “Companies are always worried that they are not innovative enough and that someone else is more innovative than they are,” he remarked. On the other hand, innovations can be expensive and risky endeavors. “I’ve seen a reticence of companies to pick up early-stage stuff because they’re either hedging their bets or they’re being cost conscious because the margins are tight.”
Hanumara explained that companies are set up to maintain the status quo so they don’t lose market share, to make incremental improvements, and to bring new products to the market, provided those products “are in a space they already know.” He said, “I would ask companies to be a little more open to the unknown, to take a look at some of the early projects, and to come on board and see if they can support and guide a project into a commercially viable product.” At the moment, he doesn’t have any “great examples,” but he noted, “I am working on convincing companies to do so.”
4. Carry a Torch
Innovation doesn’t happen overnight. The idea for a product to cool the saline on ambulances, for instance, was a good one, but it languished five years until a group of graduate students in the joint department’s graduate-level medical technology and design course resurrected it. They felt it had potential and developed a business model for it, explained Andrew DiMeo, Sr., Ph.D., assistant professor of the practice, and the department’s director of industrial relations. At about the same time, Voiers was thinking about beginning a medical-device start-up company on his own. He also serves as an executive coach for the course.

Voiers considered about a half dozen ideas, and used the following screening questions to whittle down the pool:
- Is there a clear unmet medical need that doctors/caregivers immediately recognize?
- Do I care about the medical need?
- Is the market size large enough? (He was specifically looking for a total addressable market of US$500 million or larger.)
- Is there a simple, straightforward regulatory path? (He was searching for 510(k) or 510(k)-exempt opportunities.)
- Has proof of concept been demonstrated?
- Is the technology patentable?
He ultimately settled on the saline-cooling technology, because it not only met all the criteria, but also was the only opportunity that had pre-existing clinical data to support the product. “The availability of clinical data was really the clincher for me,” he said. After licensing the technology from the university, both he and DiMeo began building the company Novocor Medical Systems around the product, which is now called HypoCore. The product has passed through proof-of-concept prototypes, and the company is now constructing its first commercial usable prototype.
“In all,” DiMeo said, “it took a combination of innovative product development and invention disclosure combined with good business modeling, multiple classes looking at it, and a torchbearer who was willing to hang onto the thing all that time before it got to the point where it was ready for angel financing.” In this case, DiMeo became the torchbearer, the person who just wouldn’t let the idea fade away.
A product’s champion can be the inventor, a clinician, a technology transfer officer, a businessperson who is looking for something new, or any number of other people who may have an interest in the technology. “It takes a great deal of entrepreneurship to carry one of these ideas over what can potentially be several years,” DiMeo commented. “Innovation really comes down to someone who is committed to carrying a torch.” For the cold-saline project, Dimeo took on that role because he wanted to show his students that it was indeed possible for one of their products to make it to the marketplace and have an impact on society.
Sometimes the torchbearers are the inventors themselves, such as Thomas Lipoma and Carson Darling, two students who went through the medical device design course at MIT two years ago, recalled Hanumara. Lipoma and Darling, joined by two other MIT students, cofounded Rest Devices based on their class project: a sensor-outfitted shirt to noninvasively monitor respiration patterns and diagnose sleep apnea in adults. “We knew pretty early on that we wanted to start a company, so while we were still undergraduates, we were actively looking for ideas and projects,” Lipoma said. They settled on the sleep shirt because it had both strong market traction and a potentially large market.
The four began working full-time on the company, and shortly after they graduated, the idea had progressed sufficiently to attract outside funding: US$500,000 by mid-2011. The company now sells the adult sleep shirts to researchers, but its primary product has transformed into a “onesie,” called the Mimo, which is a one-piece infant outfit that serves as a baby monitor.

Like the diagnostic adult shirts, the onesies are based around “these really cool, really thin, vinyl respiration sensors that we have. When we press them onto any kind of garment, they can pick up respiration through the movement of the chest,” Lipoma said. Besides the sensors, which are on the outside of the material and therefore have no contact with the skin, the onesie comes with a small plastic turtle that attaches with magnets to the garment and relays the sensor information to a base station. “The combination of the turtle and the sensors provides parents with real-time information about breathing, skin temperature, body position, and movement to determine whether the child is asleep, awake, too hot, too cold—pretty much everything,” he said. Algorithms run in the background to watch for such potential concerns as a pause in breathing or the baby rolling onto his or her stomach, and can send alerts to a parent’s smartphone.
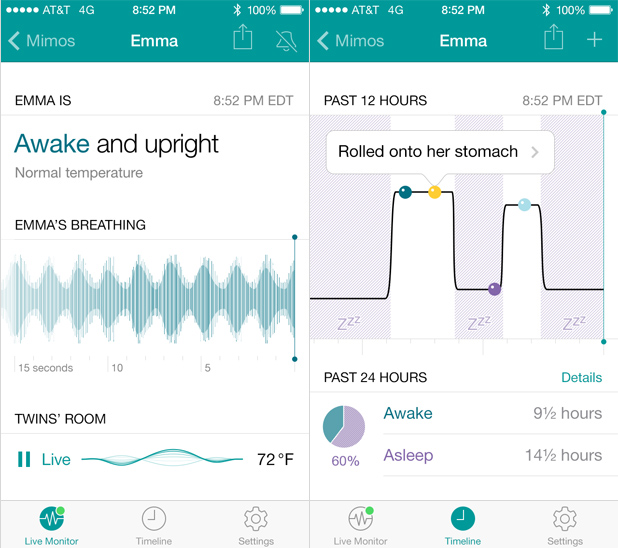
The four MIT alumni have put in considerable time and effort to bring this project to fruition. As the payoff for their dedication to that initial idea, the Mimo begins shipping nationwide in early 2014.
5. Don’t Go It Alone
Inventors do not have to venture into the unknown alone. Centers are popping up across the country to bring together all of the players that make innovations possible. One is the Global Center for Medical Innovation (GCMI), an independent, nonprofit organization in Atlanta that was founded by leading research and academic centers in Atlanta, including Georgia Institute of Technology (Georgia Tech).
“With Emory University, Georgia Tech, and other research universities throughout the region, the medical device industry here should be bigger,” said Tiffany Wilson Karp, general manager and chief operating officer of GCMI. “And when you look at the fundamental reasons why, it’s that someone comes up with an idea, but they aren’t sure what to do next. For example, you might have a physician with a great idea to improve hospital efficiency or to improve patient care, or an academic researcher who’s got a great new medtech invention, but they may not necessarily know what it takes to get a their idea to the market.”
GCMI is designed to assist with those topics that can be difficult for innovators to navigate, she said. That includes regulatory pathways and strategies, the intellectual-property landscape in the context of the industry, the wants and needs of investors, and the investment potential of a product. GCMI also includes a 12,000-ft2 medical device commercialization center complete with full design engineering and prototyping capabilities, sophisticated three-dimensional printers, a complete machine shop, and validating clean room space. The commercialization center opened in April 2012 and came on the heels of an i6 Challenge grant the GCMI won from the U.S. Department of Commerce in 2010.
“In addition, we’ve got an ever-growing network of industry experts that we know and trust. So when someone comes in with an idea or has a question they need answered, we know whom to call or to bring in to answer critical questions and accelerate the commercialization process,” Karp said, noting that the center’s network includes academic researchers, physician entrepreneurs, small start-up companies, and new-concept groups throughout the industry. Investors are also part of the network. “We have ongoing dialogues with investors to find out what technologies they’re interested in, what types of companies they might invest in, various levels of funding, and how they want ideas pitched.” All of that helps the inventor tell their story in the best light, she said.
Michigan’s University Research Corridor (URC) has a slightly different premise. It is an alliance between U-M, Michigan State University, and Wayne State University, which when combined, account for about 94% of all the academic research and development (R&D) taking place within the state of Michigan, said Jeff Mason, URC executive director. “Last year, more than US$2 billion in R&D went on within these three research institutions,” he said.
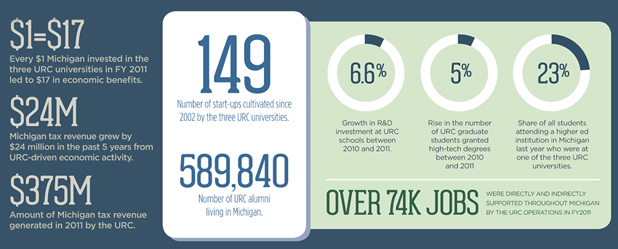
In essence, the URC serves as a marketing center that brings attention to the state and its R&D capabilities, potentially stimulating new research and encouraging commercialization. It also fosters collaborations so that faculty and researchers can join forces on common research interests or share equipment, Mason said.
Single-university centers have also joined the innovation-promoting bandwagon. “The MEDRC brings together people from medical device and microelectronics companies, physicians and clinicians from Boston hospitals that are just across the river from MIT, and technologists from MIT,” Sodini said. “We include all three because it takes all three. Doctors or clinicians know exactly what they want, but the volumes on those products are often very small, usually just for their patients. For technologists, their technology is the only thing that’s important. And from the industry standpoint, they just want to have something they can sell and sell at volume, especially in microelectronics. So the center pools all of these together.”
That type of merger works, Sodini said. The proof lies in one of his research group’s own projects, a vital signs monitor that is worn at the ear. “It measures your electrocardiogram (ECG), for both heart rate and timing information; it measures your ballistocardiogram (BCG), which is a mechanical signal that occurs when the blood is expelled out of the left ventricle and into the aorta; and the third signal it picks up is the photoplethysmogram (PPG), which yields oxygen-saturation level and the time of arrival of the pulsatile,” Sodini explained. “This allows us to use timing information from two or more of the signals to acquire cardiac parameters such as the pre-ejection period, heart contractility, and cardiac output, or even estimate the mean arterial blood pressure.”
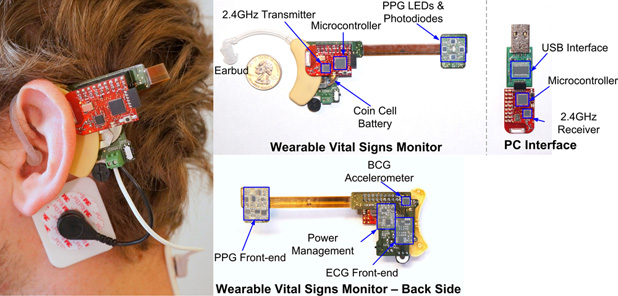
The project couldn’t have proceeded without collaboration, he noted. The prototype development required an understanding of the physiology from the medical side and knowledge of the electronics from the engineering side, and layered on top, a start-up company is handling the commercialization.
Strong collaborations just make good sense because each partner brings something different to the table, commented Hanumara. “For instance, academia is really good at ideation and industry is fabulous at execution,” he said. “Companies are always worried that they aren’t innovative enough. They see all of our new ideas, and they wonder how we can do it. The answer is simple: It’s because we don’t do what they do well.”
6. Be Flexible
Sometimes, the development direction may need to take an unexpected turn, especially when it’s important to bring in some cash flow while waiting for a medical device to go through clinical testing and the regulatory approval process.
In the case of Rest Devices, the shift from the adult shirt to the onesie came for a few reasons. One was that the diagnostic adult shirt is designated by the U.S. Food and Drug Administration (FDA) as a class-one medical device and therefore must go through the FDA approval process, which includes clinical testing. That takes time—as much as five years, Lipoma estimated—and costs money.
Still, they didn’t view the FDA approval as an insurmountable obstacle. The cost is high but not extreme; the pathway is rather long but relatively straightforward; and consultants are available to help start-ups through the process, he said. The bigger complication was insurance reimbursement. “Who’s going to pay for it? How are they going to pay for it? There are so many variables at play, so much politics around insurance reimbursement, and it’s all changing so quickly right now that it’s almost impossible to predict where the entire marketplace is going to be after you do get FDA approval,” Lipoma said. “There are a lot of problems that need to be fixed before we will feel really confident as a start-up making a medical device.”
With all that in mind, the onesie made more sense as the company’s initial product. Since it is a consumer product and the company is not making any medical claims, it doesn’t need the FDA nod and sales can begin immediately. And by selling directly to consumers, insurance reimbursement isn’t involved.
That’s not to say a medical device is not in their future, he said. “Ideally, we want to be the first hospital–home product, so if your child is coming out of the hospital with a onesie, we think it should be the Mimo onesie so you can track your baby,” he said, and that will demand FDA clearance. “We’ll have to validate that our product is doing what we say it is at a clinical level.”
For now, though, Lipoma and the other 20-somethings running Rest Devices are happy to have the flexibility to continue their aspirations toward a medical device, while also putting out a consumer product that will give their bottom line a boost.
7. Understand the Barriers
Money is always an issue, and it can be a barrier to biomedical innovation. “Venture capital or angel investors are not quite ready to invest in university research at an early stage, and unfortunately, available funding sources to bring it from research endeavor to a product that’s ready to spin out of the university are very small,” DiMeo said. That means it may be months or even years before enough funding is cobbled together to move a project forward (see “An Investor’s Point of View”).
[accordion title=”An Investor’s Point of View”]
What exactly are investors looking for in a new technology? That depends, according to Bob Crutchfield, the general partner for Harbert Venture Partners’ (HVP) health care practice. HVP of Birmingham, Alabama, is an institutional venture capital investor firm that sponsors alternative asset investment strategies, including those in the biomedical arena. “We are early-growth-stage investors, so what we’re looking for before we deploy capital is evidence of market adoption demonstrated by revenue, which means customers,” he said.
HVP doesn’t invest until a market-launch product is ready, Crutchfield said. “We want to see the regulatory processes completed and approvals received in most cases. An alternative would be having a surrogate for revenue.” He provided two examples.
In one, HVP invested in nContact Inc. of Morrisville, North Carolina, which developed a device to treat atrial fibrillation in a far less invasive way than the standard, aggressive surgical procedure, Crutchfield said. “The beauty of this product was that they already had FDA approval, they were already in the market, and they had a couple of customers who were early adopters and were using the product. So we came in and put capital to work to grow and expand the business,” he said. “Today, that product is beginning to be recognized as the leading solution to treating patients with atrial fibrillation.”
In the second example, Crutchfield described HVP’s investment in Intelliject of Richmond, Virginia, which developed Auvi-Q, an epinephrine-injection device for emergency treatment of life-threatening allergic anaphylaxis. “One of the things with Intelliject that was very appealing to us was its management team, including the inventors who created a very elegant solution to the delivery of epinephrine,” Crutchfield said. He described the device as an easy-to-carry and easy-to-use automated delivery platform that has a large and growing market. “What we also liked was that it had capabilities for using other types of drugs, so there was expandability.”
HVP got involved rather early in the development stage of the Intelliject product, Crutchfield said, because it had a surrogate for revenue. The surrogate was a corporate partner, French pharmaceutical giant Sanofi, which agreed to “take the product to market if we could get it through the regulatory pathway,” he said. It has worked out well. “We’re having really great success today.”
Overall, Crutchfield advised research universities, which he said are doing the vast majority of the research behind new biomedical products, to take several steps to entice investors. University offices of technology transfer and licensing must not only view licensing as a means of creating monetized value for technologies under development, but also as a means to nurture those technologies that have potential for becoming operating companies, he said. For the latter, universities must provide “the right level of support, management, guidance, interactions with the business community at the appropriate times, and help in positioning technologies to get them through proof-of-concept prototype development phases. That provides clarity around what the addressable market is and what the regulatory hurdles are. That’s very important to an investor,” he said.
Most venture capitalists today want to invest with an eye toward growth equity, Crutchfield said, and aren’t interested in putting their money into initial research. That means universities need to take a more active role in bringing research projects further down the commercial pathway so that they’re closer to market readiness. “If universities become more focused on the economic development aspects of commercialization—where they’re actually trying to facilitate the building of companies—then I think you’ll see investors potentially moving downstream again and maybe coming into these deals earlier.”
[/accordion]
Nisbet agreed that money can be a major impediment. “Most of the basic research here at U-M and at other research universities is funded by federal, state, or university sources, but there are fewer options to fine-tune the research and get it to a point where an investor would be interested.” Technology transfer offices like his, as well as innovation centers, can often help either with grant-submission assistance or direct funding. Nisbet’s office, for instance, provides some of that necessary funding, along with access to talent to address key commercialization issues.
While the lack of funding can and sometimes does stop technology development in its tracks, insurance reimbursement can become an even bigger barrier once a product reaches the commercialization stage, Lipoma explained. “It’s easy to think of the FDA as the only problem, when in reality for a lot of different devices and a lot of different companies, insurance is going to be a much bigger issue down the road.”
Insurance reimbursement is in dire need of an overhaul in the United States, said Carol Lewis, associate director of the UNC Health Care System’s Center for Innovation. The center’s main objective is to promote innovation in health care delivery across the UNC Health Care System and the UNC School of Medicine. “We have these wonderful disruptive innovations that are good for the patient and good for the payers because they’re less expensive overall, but they are not necessarily financially supported through reimbursement models. So, we end up spending a lot of time talking with collaborators to help us fund some of the innovations, either through in-kind contributions or even direct funding to launch pilot projects that will demonstrate the value of these innovations.”
Karp identified the same obstacles. “There’s still an issue in the difficulty in getting innovations through the FDA, but I think that it’s getting better. Health care reform, and specifically reimbursement and regulatory uncertainty, remain critical areas that we need to address as a country for investor dollars to start flowing again.” She added, “Until we really address those fundamental, macro issues, I think we’ll see continued tightening of the purse strings for investments in their early stage, and that is really hurting innovation in this country.”
Measuring Success
The demand and the drive for health care innovation is stronger than ever, and with so much excitement over new discoveries and products, the next quandary is how “innovation” can be measured. “That’s a really good question and an especially tough one to answer for the ones planting the seeds,” DiMeo said. “At universities, you’re seeing more metrics on the number of spinout companies, whereas in the past, technology transfer was measured more in cash, such as royalties, brought in. Of course, the next question is: Does that mean good, successful spinouts? That’s going to be hard to say until you get down to measurements on such things as jobs created.”
Success stories are one way to measure the impact of innovation, said Nisbet. Examples of university research that has spawned start-up companies over the years are many, including recent start-ups in Ann Arbor, Michigan, such as Tissue Regeneration Systems Inc., which is creating a skeletal reconstruction and bone regeneration technology platform that it licensed from U-M and the University of Wisconsin, and Histosonics Inc., which is using U-M technology to develop noninvasive, image-guided therapeutic ultrasounds for benign prostatic hyperplasia (BPH, or benign enlargement of the prostate) and other conditions. “If you want to talk about the impact of innovation, the best way to describe it is with stories,” he said.
UNC’s Center for Innovation measures the success of its individual projects one at a time, Lewis said. “Each project has its own set of outcomes, which have to involve both cost improvement as well as quality-of-care improvement.” Measuring the success of the center is a little thornier, she said. “In that case, we’re looking at the number of innovations we’re supporting; we’re tracking dollars that we’re bringing in from outside sources; and we’re trying to figure out how to gauge our impact on the organizations culturally, and that’s very difficult.” Fortunately, she said, UNC and the affiliated UNC Health Care System are already leaders in innovation, so they have quite a high starting point.
“We know that health care innovation is critical,” Lewis said. At the center, innovation efforts are “aimed at reducing the overall cost of care while improving quality at the same time, so we’re focused on all kinds of disruptive innovations related to care-delivery models and the payment models that support them,” she said. That includes biomedical devices as well as innovations involving more effective and efficient use of the workforce, coordination and transition of care, and new kinds of payment models. “We’re really looking across the board,” she said.
Lewis also noted that new opportunities are around nearly every corner. “Technologies involving social media, patient engagement, and changing patient behavior are just a few. There are so many untapped areas where technology can really help to transform health care. That’s why the Center for Innovation is here and has support.”
The center also launched a program called Bright Ideas for Health and, in March 2013, put out a very general challenge to employees using a social media/crowdsourcing tool to identify unmet needs and potential solutions. “We got back more than 100 good ideas and floated a handful up to the top as winners. At this point, we’re working to implement some of the ideas that had the fewest barriers to implementation. It was a very fun and interesting exercise, but one of the challenges with this kind of crowdsourcing is that while you might get a lot of good ideas, if you don’t have the funding or the buy-in from the leaders in that area to then do something with those ideas, what have you really accomplished? We’re trying very hard to have that closure on the back end. That’s important.”
She said the center is also trying to gauge the internal culture for innovation at UNC. “We’re looking at surveying our own employees to find out how innovative they feel we are as an organization and whether they feel they can bring innovation to their areas.” While UNC is certainly no stranger to innovation, she said there’s always room for improvement. “A culture of innovation is a pillar that we must support.”
When all is said and done, the definitive metric for success lies at the heart of health care innovation, according to DiMeo. “Ultimately, it’s going to come down to patients’ lives saved or number of patients’ lives positively impacted. Whether the innovations are coming out of universities or companies, at the end of the day, the important thing is that we’re enhancing the health and quality of life of patients.”
DiMeo added, “If you can improve health outcomes, that’s innovation. If you can reduce the costs of health care, that’s innovation. And if you can do both of those things, then that’s truly something innovative.”



