Light therapy, nanotechnology, and transcutaneous electrical acustimulation offer surprising benefits in the quest to diminish pain
For the one in five adults who suffer from chronic pain, treatment largely relies on pharmaceuticals, which can come with serious downsides [1]. “They mitigate pain, but in the long term lead to such significant side effects and consequences that the burden from the treatment many times starts to exceed the burden from disease, and it attacks the person’s quality of life,” says Padma Gulur, M.D., professor of anesthesiology and head of the Pain Relief and Opioid Mitigation Innovation Science (PROMIS) Laboratory, Duke University (Figure 1).
Additionally, opioid medications, which are some of the most effective treatments for pain available for physicians, also place patients at risk of opioid use disorder. “The burden on the patient, the burden on their families, on society as a whole is just immense from these medications,” says Gulur.
This means there is an incredible need for new treatments for pain patients, leading clinicians and researchers like Gulur to step outside the box in imagining new treatment strategies that could one day improve the quality of life for patients living with chronic pain.
Seeing the light
Gulur is particularly interested in adding nonpharmacological pain relief strategies to the pain clinician’s toolkit. “If you have to use a combination of things, most of us would prefer those that come with no side effects,” she says.
While there are currently available nonpharmacological treatments for pain, including cognitive behavioral therapy, acupuncture, and massage therapy, these options often require a large time commitment to keep up the pain relief—unlike taking a pill. “That’s one of the reasons you’ll see that even though these therapies exist and studies show there are reasonable results with many of them, you’re just not seeing the uptake or the consistent use of them,” says Gulur. “It’s just not convenient.”
Inspired by the calming effect of water and forests, Gulur began to explore the effect of blue and green light on pain patients. “It started to become obvious that the visual perceptions that we have—our visual circuits—impact other neuronal circuits in our brain,” she says. Research studies from different groups suggest that green light, in particular, may have pain-relieving properties. In rats, green light can have long-lasting effects on both acute and chronic pain [2], and studies in humans have found that green light may alleviate migraines [3] and improve fibromyalgia pain [4].


Instead of asking patients to stay near a blue or green light for a period of time, Gulur decided to go with a more user-friendly option in her first clinical trial: glasses [5] (Figure 2). “Just about everybody at some stage in their life wears glasses or sunglasses,” she says. “Pick them up, put them on. Very easy.”
Gulur and her team randomly assigned people with fibromyalgia-related chronic pain and people who were experiencing acute pain post-surgery to wear glasses that provided their eyes with blue light, green light, or full-spectrum light (the control condition) for 4 hours every day while they went about their lives for either two days—for the surgical patients—or two weeks—for the patients with chronic pain.
Results from this small trial were promising—especially for the patients assigned to wear the green glasses. While some of the patients in the control group dropped out because of headaches or not wanting to wear the glasses, many of the patients assigned the green light glasses did not want to give them back at the end of the study. “They wanted to continue using it,” says Gulur. “So at a very pragmatic and practical level, we realized this was making an impact.”
Gulur says the study proved that the intervention was feasible and that patients were willing and able to wear the glasses for 4 hours a day. “It was easy for them,” she says. “We did ask people if they were concerned in any way to walk around with green glasses or blue glasses and they actually thought it was cool.”
While the study showed some strong trends pointing toward clinical improvement, a larger study will be needed to prove whether these effects are statistically significant and to test for safety and efficacy. “Number one, we want to statistically see consistent reduction in opioid use,” says Gulur.
She also wants to explore the mechanism of action underlying these effects, determine whether there is a dose effect, and figure out what particular wavelengths of green light are most effective. “It could be that for certain disease types or individual patient characteristics, one wavelength focus band is better than the other,” she says. “There’s so much more refining we can do, but it’s exciting.”
Gulur notes, however, that sometimes it can be difficult to secure funding for research on nonpharmacological strategies for pain. “I’m really hoping that we can take this science to another level because this is showing just so much potential,” says Gulur. “This is one of the most promising things I’ve seen in a long time.”

Ultrasonic ketamine uncaging for chronic pain
To avoid an overreliance on opioid medications some physicians are prescribing ketamine for chronic pain. Unfortunately, ketamine can come with its own side effects such as sedation, dissociation, and other psychedelic effects. Raag Airan, M.D., Ph.D., assistant professor of radiology at Stanford University, is developing a technique to target the release of ketamine directly in pain circuits within the brain (Figure 3). In particular, he is targeting a part of the brain called the anterior cingulate cortex (ACC).
“The hypothesis here is that if we directly infuse ketamine—or at least bias our delivery of ketamine—to the ACC in patients with chronic pain, we can hopefully maximize its efficacy for chronic pain without necessarily inducing all of those other effects,” says Airan.
To do this, Airan’s team has developed a way to “cage” ketamine—and other drugs—within a biodegradable ultrasound-sensitive nanoparticle shell [6] (Figure 4). After the caged ketamine is administered intravenously, ultrasound waves applied to a particular brain region, like the ACC, can change the conformation of the cage, releasing the ketamine to act on local neural circuits.
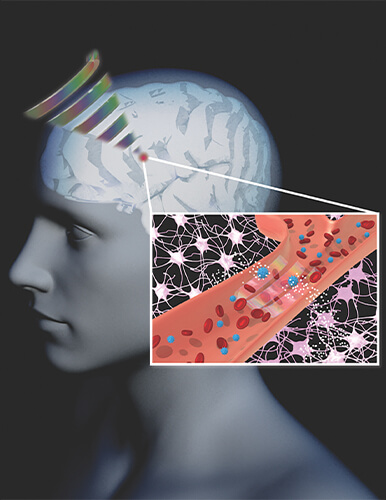
In rodent studies, Airan’s group has been able to observe that the technique works by applying ultrasound waves to a brain region analogous to the ACC and measuring electroencephalogram (EEG) signal changes. “We get reasonable looking effects in the rat that [suggest] in fact we are inducing selective release and that it’s having a neural effect,” he says. In addition, he says the EEG changes in response to the targeted ketamine delivery appear to be stronger—and act on a different EEG band—than changes seen with regular intravenous administration of ketamine.
The next step is to see whether a similar effect occurs in humans. Airan has validated a protocol for pharmaceutical-grade production of the nanocarriers. The first human trial will focus on safety. “Fortunately, by design, the component materials of our formulation have been used in humans,” he says. The study will also use EEG to monitor changes in brainwave activity in response to the ketamine. Future studies will then focus on how well this translates to pain relief in patients.
Airan says he can also see potential for the technology to be used diagnostically—perhaps to determine which chronic pain patients are likely to respond to an ACC-based intervention. Additionally, it may have a future use as a scientific tool that allows researchers to tease apart the effects of drugs on specific parts of the nervous system.
Ultrasonic uncaging of neuromodulators may also be useful for treating other nervous system diseases and disorders. In another study, Airan’s group was able to use ultrasonic uncaging to deliver the anesthetic agent propofol to selectively shut down the activity of millimeter-sized brain regions [7]. The group is now seeing whether this technique may be useful for treatment-resistant epilepsy. They are also interested in using ultrasonic uncaging with other psychedelic compounds. Fortunately, the chemistry of these drugs makes them good candidates for being encased in the nanocarrier. “We see increasing evidence that they have therapeutic psychiatric benefit,” he says. “How does this work? How can we make it work better? Not everyone really wants the psychedelic experience just to get therapy. Is it more efficacious if we separate that?”
Acustimulation for IBS pain
Some pain conditions particularly need new treatments. One of the main symptoms of irritable bowel syndrome (IBS), which affects around 11% of people across the globe, is lower abdominal pain [8]. “At this time, there are no dedicated medications for treating abdominal pain in patients with IBS,” says Jiande Chen, Ph.D., professor of medicine at the University of Michigan Medical School (Figure 5). Current treatment usually relies on antidepressants, which do not always work well for IBS pain and can have unwanted side effects.
Some groups are exploring the use of implanted devices to target IBS pain by directly stimulating the sacral nerve, which innervates the colon, but this is not a great option for all people with IBS. “If you ask a patient to have a surgery, although it’s minimally invasive, a lot of patients have hesitation,” says Chen. Additionally, noninvasive pain devices that work by stimulating muscle near a pain site do not work for pain in the stomach or colon.
The lack of treatment options led Chen to develop a novel noninvasive technique for treating IBS pain. “The main idea actually originated from traditional Chinese acupuncture,” says Chen. While acupuncture can be used to relieve IBS pain, it only works for a few hours. “It has to be practiced by an acupuncturist or by a doctor, and the patient has to come to hospital almost every day,” he says. This makes it impractical for IBS patients.

Chen’s new technique—transcutaneous electrical acustimulation (TEA)—uses surface electrodes to administer electrical stimulation at particular acupuncture points [9]. It works by targeting specific nerves through the skin. “I combined traditional Chinese acupuncture with our modern theory of neuromodulation,” says Chen. Because TEA uses electrodes on the skin to deliver pain relief, patients are able to self-administer the stimulation themselves at home.
TEA can be used at traditional acupuncture sites near peripheral nerves that innervate the gastrointestinal system. For example, ST36 is an acupuncture site below the kneecap near three nerves that connect to the sacral nerve. Besides stimulating the colon via the sacral nerve, Chen says acustimulation at this site may also change the way the brain processes pain.
Early results suggest that TEA can suppress the activity of the sympathetic nervous system—which is involved in pain—and enhance parasympathetic activity. It also reduces signs of inflammation, which plays a major role in pain in IBS patients, says Chen.
A 2021 study of 42 IBS patients found that those who were randomly assigned to receive TEA stimulation of their vagal nerve applied through their ear for four weeks had decreased IBS symptoms, pain, and markers of inflammation, as well as improved quality of life and enhanced vagal activity, compared to those who received sham stimulation [10]. Stimulation of the vagal nerve was chosen because it innervates the esophagus, stomach, small bowel, and part of the colon. In the future, Chen says physicians may be able to target a specific site for TEA depending on where patients tend to feel their IBS pain.
Chen is currently in the middle of two feasibility studies for using TEA to treat IBS pain. “I’m doing more mechanistic research and really trying to understand how exactly it can desensitize the organ or can be sent inside the brain,” he says. “If I know this better then we can improve the methodology.” Some important questions that remain are how long the effects of TEA last, how frequently it should be administered, and whether—after a period of treatment—TEA can actually make IBS pain disappear for a while. Chen is also exploring whether TEA could be used to treat pain in other conditions such as functional dyspepsia, heartburn, acid reflux, and fibromyalgia.
In hopes of making TEA more user-friendly, Chen is currently working with companies to develop skin patches and earbud-like devices for administering the stimulation. “You have to make a device very comfortable so patients are willing to use it,” he says.
Chen hopes more engineers will team up with researchers and clinicians to develop novel treatments for pain, particularly pain associated with gastrointestinal problems like IBS. “The potential actually is great,” he says. “We need a lot of engineers to help develop good therapies. Pain does not kill people, but pain affects the quality of life.”
References
- CDC. (2020). Products—Data Briefs—Number 390—November 2020. Accessed: Jan. 20, 2022. [Online]. Available: https://www.cdc.gov/nchs/products/databriefs/db390.htm
- M. M. Ibrahim et al., “Long-lasting antinociceptive effects of green light in acute and chronic pain in rats,” Pain, vol. 158, no. 2, pp. 347–360, Feb. 2017.
- L. F. Martin et al., “Evaluation of green light exposure on headache frequency and quality of life in migraine patients: A preliminary one-way cross-over clinical trial,” Cephalalgia, vol. 41, no. 2, pp. 135–147, Feb. 2021.
- L. Martin et al., “Green light exposure improves pain and quality of life in fibromyalgia patients: A preliminary one-way crossover clinical trial,” Pain Med., vol. 22, no. 1, pp. 118–130, Feb. 2021.
- Clinicaltrials. (2022). Opioid Sparing Potential of Light-Induced Analgesia—Full Text View—ClinicalTrials.gov. Accessed: Jan. 20, 2022. [Online]. Available: https://clinicaltrials.gov/ct2/show/study/NCT03890419
- J. B. Wang et al., “Focused ultrasound for noninvasive, focal pharmacologic neurointervention,” Frontiers Neurosci., vol. 14, p. 675, Jul. 2020.
- J. Wang et al., “Noninvasive ultrasonic drug uncaging maps whole-brain functional networks,” Neuron, vol. 100, no. 3, pp. 728.e7–738.e7, 2018.
- T. Card, C. Canavan, and J. West, “The epidemiology of irritable bowel syndrome,” Clin. Epidemiol., vol. 6, p. 71, Feb. 2014.
- J. Cheng et al., “Potential of electrical neuromodulation for inflammatory bowel disease,” Inflammatory Bowel Diseases, vol. 26, no. 8, pp. 1119–1130, Jul. 2020.
- X. Shi et al., “Ameliorating effects and mechanisms of transcutaneous auricular vagal nerve stimulation on abdominal pain and constipation,” JCI Insight, vol. 6, no. 14, Jul. 2021, Art. no. e150052.