Imagine a natural disaster, such as an earthquake, causing residential buildings to collapse and trapping the people in- side underneath the rubble. Over the following days, first responders spend a significant amount of time locating survivors. Despite having the assistance of life-detection tools and techniques, these first responders are still hampered by certain limitations in searching for survivors and pinpointing their exact location deep within the rubble. Current methods involving dogs and relatively large robots and machinery can assist only with surface-level surveying of the disaster area and removal of rubble. In contrast, a swarm of centimeter-scale robots could be employed for under-rubble mapping and surveying in these scenarios. In fact, progress has been made in developing centimeter-scale robotics that could assist first responders in the future. However, these prototypes still require improvements in the efficiency of the mechanical parts and control systems deployed in real-life scenarios.
Some of these inefficiencies could potentially be addressed by using live insects instrumented with electronic backpacks as a low-cost, alternative solution. For example, Madagascar hissing cockroaches can be transformed into roach biological robots (biobots), or roach-bots, for investigating deep within building rubble (Figure 1). These insects are commercially available and, like most roaches, possess good navigational and locomotive capabilities. As such, they would be useful as instrumented beasts of burden, traveling through crevices in dynamic environments such as in the rubble of postdisaster scenarios and other premises hazardous to humans and other mammals.
![Figure 1: An illustration of a cyberphysically organized sensor network of roach-bots; grayed out roach-bots are positioned under rubble. A process flow diagram of a roach biobotic system is shown at the top right [3].](https://www.embs.org/wp-content/uploads/2017/09/bozkurt01-2729413.png)
Biobot Research
Research progress in the field of neural engineering has enabled the development of insect-based biobots [1]–[4]. Work on aerial biobots has included flight initiation, turning, and flight cessation using Carolina sphinx moths and a species of the flower beetle. This was followed by efforts with terrestrial insect biobots using Madagascar hissing cockroaches, discoid cockroaches, and huntsman spiders. Our research team at North Carolina State University has been able to precisely control the locomotion of the Madagascar hissing roach-bots by remotely applying selective neurostimulation to the antenna and cerci using implanted electrodes, and a low-power electronic backpack constructed with off-the-shelf components and modern system-on-chip devices (i.e., CC2530 from Texas Instruments) [3], [4].
Methodology
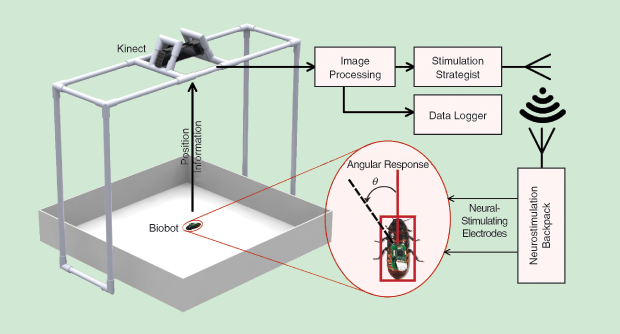
The backpacks weigh about 2 g including a 50-mAh lithium-polymer battery, which is well within the average payload capacity of adult hissing cockroaches [3]. If a stimulus is applied to the right antenna, the biobots turn left as a result of their obstacle avoidance reflex, and vice versa. Cerci stimulation triggers an escape response and makes the biobots move forward. Our team has developed an automated evaluation platform for biobotic control using a Kinect camera and custom-built computer software (Figure 2) [3]. This platform enables objective performance assessment through an automated routine that applies stimulation and then tracks the biobots’ trajectory. Using this system in controlled laboratory environments, we were able to make biobots follow follow lines (Figure 3), go through mazes (Figure 4), and be autonomously guided to a sound source using on-board microphones—a technique that may prove instrumental when locating survivors calling out for help.
![Figure 3: The paths taken by roach-bots in ten automated line-following trials [3].](https://www.embs.org/wp-content/uploads/2017/09/bozkurt03-2729413.png)
![Figure 4: A maze arena showing the designated path, with an overlay in red of the path taken by a roach biobot [4].](https://www.embs.org/wp-content/uploads/2017/09/bozkurt04-2729413.png)
Cyberphysical Infrastructure
A cyberphysically organized sensor network can be established with these roach-bots [4], employing an unsupervised search strategy in an under-rubble scenario. Each of the roach-bots can be made to move randomly within a designated region [4] while mapping that region and locating any relevant signal of interest. Positional information from each roach-bot can be evaluated using received radio signal strength and inertial measurement units [4]. This could provide a cyberphysical infrastructure for information gathering and subsequent sharing with first responders, thereby helping to save crucial time.
Achieving Reliability
To achieve such a sensor network through the biobots’ electronic backpacks, the reliability of the biotic–abiotic interface between the backpack and the insect must be ensured to avoid failure in real-world settings. A gradual inactivation or a decrease in biobotic response to neurostimulation has been observed in some laboratory-based experiments [5]. We hypothesize this to be caused by a temporary or permanent inefficiencies of the bioelectrical coupling occuring at the insects’ tissue–electrode interface, resulting from the delicate nature of the charge transfer process.
For efficient biobotic control, the research questions that need to be addressed include
- how the design of the implanted electrodes and the pulse-application strategy affect performance efficiency over time and under various conditions
- how the electrode design and pulse shaping affect the tissue–electrode interface
- whether these factors can be reflected in our automated evaluation platform to further optimize the roach-bot control strategy.
We are in the process of analyzing the material of the implanted electrodes, the quality of the tissue–electrode interface, and the resulting behavior of the roach-bots.
Assessment Model to Optimize Control
In earlier experiments, we used commercially available stainless-steel wires as stimulation electrodes. Although very practical and inexpensive, the stainless steel suffers from a low charge-injection capacity and needs to be replaced with microfabricated electrodes carefully designed for biobotic neurostimulation. We are in the process of performing a detailed investigation on the long-term stability of such implanted electrodes and how the efficiency of the charge-transfer process can be improved by fine-tuning the materials used.
To perform such a long-term analysis, we conducted a video-based evaluation using the previously described automated platform (Figure 2, earlier in this article). In addition to providing information on how the biobots behave, such analysis also helps in forming a baseline for biobotic experiments. In this analysis, the angular change in the position of biobots in response to neurostimulation pulses provides a quantifiable measure of biobotic control. A longitudinal assessment of this angle was performed over a set period of time. This angular change proportionally varied with the pulse width of applied stimuli. For a fixed pulse width, we have shown that the magnitude of the change gradually lessens over a course of weeks as the quality of tissue–electrode interface degrades.
Concurrently, a remote and real-time assessment of the tissue–electrode interface was performed at the electrode implantation site, using the additional circuitry on the biobot’s electronic backpack [5]. We used electrochemical assessment, including electrochemical impedance spectroscopy, to characterize the tissue–electrode interface and monitor the variation in the interface quality. With an electrical equivalent model of the interface, we further analyzed the interface in terms of the hypothetical parameters responsible for charge transfer across the interface when an applied stimulus induces it. Remote monitoring at regular intervals allows us to check the interface condition and infer how this would change over time.
Roach-Bots to the Rescue
We believe that these roach-bots would play a critical role in the development of a sensor network with synthetic or biobotic centimeter-scale agents to effectively help first responders in emergency situations after natural disasters. Here, we have presented a road map and experimental infrastructure that can address one of the most critical aspects of biobotic research: the quality of tissue–electrode interface. The careful design of neurostimulation electrodes and remote monitoring of the tissue–electrode interface’s quality at the electrode implantation site are the two most important factors that must be addressed to mitigate any potential degradation in biobotic activity, prevent tissue damage at the interface, and further optimize the control of roach-bots.
References
- H. Sato and M. M. Maharbiz, “Recent developments in the remote radio control of insect flight,” Front. Neurosci., vol. 4, pp. 199, Dec. 2010.
- A. Bozkurt, R. F. Gilmour, and A. Lal, “Balloon-assisted flight of radio-controlled insect biobots,” IEEE Trans. Biomed. Eng., vol. 56, no. 9, pp. 2304–2307, Sept. 2009.
- T. Latif, E. Whitmire, T. Novak, and A. Bozkurt, “Sound localization sensors for search and rescue biobots,” IEEE Sensors J., vol. 16, no. 10, pp. 3444–3453, May 2016.
- A. Bozkurt, E. Lobaton, and M. Sichitiu, “A biobotic distributed sensor network for under-rubble search and rescue,” Computer, vol. 49, no. 5, pp. 38–46, May 2016.
- T. Latif and A. Bozkurt, “A wireless system for longitudinal assessment of tissue-electrode interface in biobots,” in Proc. 2015 IEEE Biomedical Circuits Systems Conf., Atlanta, GA, Oct. 2015, pp. 1–4.