Research on biomaterials and related subjects has been active in Italy. Starting from the very first examples of biomaterials and biomedical devices, Italian researchers have always provided valuable scientific contributions. This trend has steadily increased. To provide a rough estimate of this, it is sufficient to search PubMed, a free search engine accessing primarily the MEDLINE database of references and abstracts on life sciences and biomedical topics, with the keywords “biomaterials” or “tissue engineering” and sort the results by affiliation. Again, even though this is a crude estimate, the results speak for themselves, as Italy is the third European country, in terms of publications, with an astonishing 3,700 products in the last decade.
Biomaterial research is intrinsically multidisciplinary and, as such, cannot be improvised. A thorough understanding of the fundamentals of chemistry, material science, engineering, and biology is of great importance throughout the stages of biomaterial design, processing, and testing. Luckily, when biomaterial research was en- hanced worldwide, the Italian scientific ground was very receptive, owing to a long tradition on chemistry, polymer science and technology, composite material development, and preclinical and clinical experimentation. At the be- ginning of the 1980s, different groups located all over the country began studying novel biomaterials and prosthesis. Today, the Italian top class research on biomaterials, tissue engineering, and drug delivery is a reality, and several laboratories are well recognized for their scientific contributions worldwide.
The intent of this contribution is to provide an overview of innovative Italian research in the area of biomaterials and tissue engineering. More specifically, we present and discuss relevant works published by Italian researchers in the last decade. Our aim is to integrate the scientific outcomes and technological advancements within the fields of biointerfaces, materials, and processes related to biomaterial engineering and processing. Finally, we also present advancements in the in vitro and in vivo testing of advanced devices and therapies.
Biointerfaces
Significant effort has been devoted to the development of biointerfaces to analyze or guide cell–material interactions. Relevant works span from fundamental investigations on biomolecule–material or cell–material interactions at the biointerface to the fabrication of bioactive nanoengineered surfaces to affect cell functions and fate.
In this regard, the adsorption of proteins on heterogeneous surfaces is of paramount importance in many fields involving the use of biomaterials. In fact, the interactions of a foreign material in a living body, for instance in a body implant, are typically mediated by proteins that may be adsorbed on the material surface, thus affecting its biocompatibility. The same considerations can be done also in tissue engineering (TE), that is, when cells are grown on an artificial scaffold.
Relevant aspects of the conformational and energetic changes related to the adsorption of protein fragments on surfaces of different wettability, including either hydrophobic or hydrophilic surfaces, have been widely investigated, as the interactions of the biological environment with the synthetic materials are of crucial importance for both the short-term response and long-term stability of an implant.
In recent years, the effect of nanometer-scale features on the surface density, structure, and activity of adsorbed proteins has been addressed by several authors seeking the key factors connecting nanomorphology to the modulation of protein adsorption and biofunctionality. In particular, changes in the local nanoscale chemico-physical properties of material surface can greatly affect cell–material interactions. Therefore, surface treatments of biomaterials were developed with the aim of controlling specific aspects of the cell behavior (Figure 1).
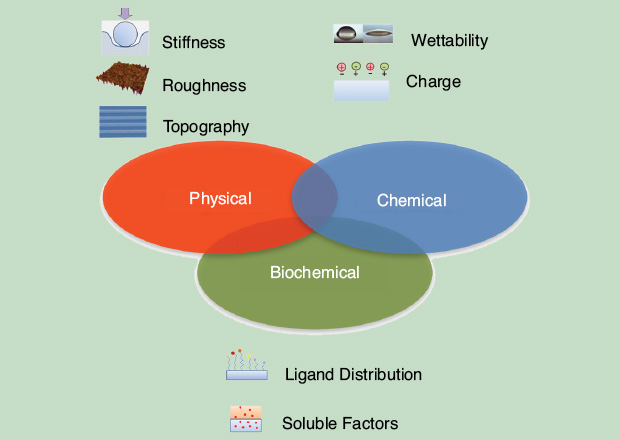
Bioactivation of material surface can be achieved with an appropriate biomolecule at interface. Small peptide sequences such as Arg-Gly-Asp (RGD) are widely used to obtain biomimetic interfaces. However, the cell recognition of bioactive ligands immobilized is strongly dependent on ligand presentation at the cell–material interface, and some skepticism has arisen on the effectiveness of such a strategy in practical contexts since serum proteins largely adsorb on synthetic surfaces. This can, in principle, alter the actual perception of a cell toward the immobilized ligands. To address this issue, it is crucial to ascertain whether a chemically conjugated integrin-binding peptide is fully recognized by a cell even if partially covered by a physisorbed layer of serum protein in more general terms. In other words, it is not clear whether competitive physisorption of protein fragments can be perceived differently from chemically anchored peptides. It was demonstrated that cells are able to discriminate, via mechanosensing mechanisms, between adhesive motifs belonging to physisorbed proteins and those firmly anchored on the material surface [1].
Topographic patterns at bio- interfaces proved to be very effective in controlling cell–material interactions. The sensing and adhesion machinery of cells are affected by topography when their interval features are tuned on the characteristic lengths of filopodial probing and focal adhesions (FAs). This can strongly alter cellular elongation and polarization. To this regard, the spatial confinement of FA growth and remodeling has a profound effect on the assembly of the cytoskeleton. This, in turn, regulates cell and nuclear shape and polarization, which are crucial in the context of mechanotransduction. Therefore, a thorough understanding of the FA remodeling on nanopatterns might allow researchers to predict the spatial configuration of the FA cytoskeleton and, possibly the cell fate, through the material–cytoskeleton crosstalk. FA confinement and modulation of their surface distribution proved to dramatically alter the cytoskeleton’s structures and dynamics, which in turn dictate cellular and nuclear shape and polarization.
Materials
The natural application of the fundamental studies on cell–material interactions at the interface is the development of functional prostheses and scaffolds for TE. Functionalized material surfaces with chemical, physical, and mechanical signals can trigger specific cell signaling or behavior, which ultimately improves the in vivo performance of the device. Conventionally, the most investigated materials fall in the main categories of metallic, polymeric, and composite materials. As for metals, material surface characteristics are mainly optimized to improve the osteointegrative properties. Indeed, developing new osteointegrative and mechanically stable coatings for titanium and titanium alloy implants is one of the primary goals for the next generation of orthopedics and dental materials. Hydroxyapatite coatings proved to enhance the osteointegration of metal implants through a tight binding to the bone mineral phase, as well as through favorable osteoblast adhesion and proliferation onto the implant surface. However, hydroxyapatite coatings are not stable and they tend to delaminate from the metal surface when challenged by the mechanical stresses experienced by the implant. A multiphase anodic spark deposition (ASD) method has been optimized, in which the formation of a thick oxide film is followed by the deposition of a calcium phosphate mineral phase and its etching by alkali.
This type of coating, BioSpark, improves the material osteointegration potential when compared to conventional ASD and offers more mechanical stability. A faster mineralization was obtained by incubation in simulated body fluids, and osteoblasts showed better adhesion, proliferation, differentiation, and collagen production. These performances were related to the surface morphology, to the film calcium/phosphate ratio and its surface oxygen content, as well as to a preferential binding of structural proteins such as fibronectin [2].
Among the polymeric materials, particular attention has been devoted to the natural polymer since it is intrinsically bioactive and synthetic polymers owing to their mechanical properties, versatility, and processability. As natural polymers, and due to their strength, native silk proteins from the silkworm have been used extensively in the medical field as suture material for centuries. Accurate positioning of fibers may recapitulate the native architecture of tissues, thus improving the mechanical and biological response of silk-based materials for in vivo implantations.
A synthetic material, polycaprolactone (PCL), a semicrystalline linear resorbable aliphatic polyester, was used as the scaffold for bone tissue engineering, due to its biocompatibility and biodegradability. However, the poor mechanical properties of PCL impair its use as a scaffold for hard tissue regeneration, unless mechanical reinforcement is provided. To enhance mechanical properties and promote osteoconductivity, hydroxyapatite (HA) particles were added to the PCL matrix. HA-loaded PCL was found to improve osteoconduction compared to the PCL alone. The results indicated that PCL represents a potential candidate as an efficient substrate for bone substitution through an accurate balance between structural/mechanical properties of polymer and biological activities.
Novel Techniques for Biomaterial Processing
The original paradigm of TE, envisaging scaffolds as temporary supporting materials only, has been largely surpassed in recent decades. As previously reported, bioactivated surfaces and scaffolds can exert potent effects on cells and surrounding tissues. However, the general concept of bioactivation goes well beyond simple biochemical functionalization. Indeed, literature highlights the role of the micro- and nanoscale structure of the substrate in controlling cell fate and functions. Therefore, several techniques have been developed during the last decade to fabricate synthetic scaffolds with specifically controlled microstructural and nanostructural features.
For example, a context in which the structure of the scaffold plays a fundamental role is represented by nerve repair. Upon injury, a local inflammatory process takes place that ultimately recruits myofibroblasts from the surrounding tissues. These cells impair the correct nerve regeneration pathway, eventually leading to a dysfunctional scar. Therefore, scaffolds for nerve TE have to deal with such a complex scenario. In other words, the optimal scaffold must not only provide the adequate stimuli for neurons and Schwann cells to set off the regeneration process, but it must also get rid of unwanted cells, thus sustaining long-term stability of the regenerated tissue. According to these observations, Harley et al. proposed a tubular scaffold for nerve regeneration constituted by a collagen-based sponge whose porosity displayed a graded pattern of dimensions [3]. Such a peculiar structure was the result of a careful optimization of the processing conditions and was aimed at pushing the unwanted myofibroblasts outward, while maintaining the correct environment for the neuronal cells to repair the injury. Such a model was recently implemented in a transected rat sciatic nerve model and provided outstanding results in terms of histology, gene expression, and myelination.
Micropositioning systems and stepper motors provided a convenient solution for the accurate spatial delivery of materials, thus allowing the production of microstructures displaying a large degree of order over an extended volume. For instance, microsyringe deposition proved to be an effective method for the production of macroscopic three-dimensional (3-D) scaffolds with a very accurate spatial resolution down to 5 mm. The system is based on pressure-controlled syringes coupled with a 3-D computer-controlled micropositioning apparatus. The system possesses an elevated versatility in terms of both material processing and scaffold design. Scaffolds with complex structures, replicating biological tissues, were produced according to this technique and cell–structure interactions were studied. We point out that microstructural features and mechanical properties are two intimately connected aspects. The possibility of delivering material elements with great spatial accuracy allows control over the gross mechanical response of the structure and, in particular, its anisotropic behavior.
While it is fairly simple to emboss nanoscale features on synthetic surfaces, the production of massive 3-D nanostructured scaffolds requires the use of specifically developed technologies. Among these, electrospinning is one of the most used because of its ease of implementation and yield. With this technology, Guarino et al. exploited the electrospinning technology to produce large nanostructured fabrics made of a composite PCL/gelatin mixture [4]. Biological assays demonstrated that such a formulation possessed excellent properties in terms of stem cell adhesion and differentiation. Interestingly, the processing procedure is also compatible with drug delivery applications.
Bottom-up technologies based on the accurate spatial assembly of individual building blocks are particularly useful when an improved spatial resolution of complex structures is required. Cell aggregates and tissue fragments self-assemble to form complex structures spontaneously. Therefore, one can develop processes to control and guide such a natural property to fabricate complex and thick tissues in vitro. Palmiero et al. reported the in vitro generation of dermal tissue starting from the self-assembly of microtissues, that is, cells cultivated in microscaffolds (Figure 2) [5].
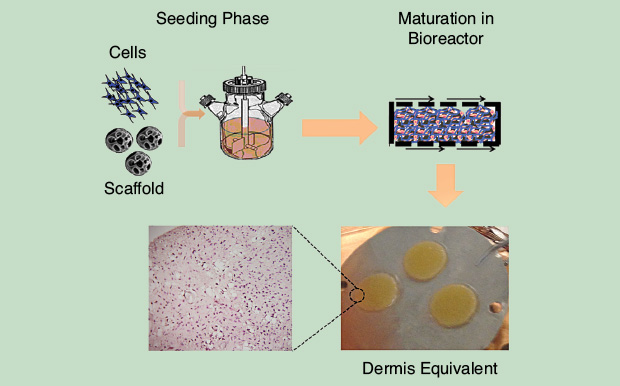
Dermal tissues thus produced displayed morphologic and mechanical features astonishingly similar to those of the native tissue. The self-assembly of microtissues also possesses the great advantage of overcoming transport limitation due to cell and tissue crowding during the in vitro culture. More recently, the same group further explored the capability of such a technique and was able to tune matrix production and assembly by modulating the microscaffold chemical/physical properties.
Polymeric and biopolymeric materials are not the only possible candidates for the production of 3-D scaffolds. Ceramic-based compounds proved to exert great influence on cell behavior, more specifically on osteogenesis and bone formation. Ceramics have been incorporated in 3-D scaffolds produced with conventional technologies. In this way, however, the ceramic material acts mostly as filler, while the main structural and mechanical properties are dictated by the polymeric template. The fabrication of bulky ceramic scaffolds with a microarchitecture resembling native bones is not straightforward. Ceramics cannot be readily processed with techniques usually employed in polymer microfabrication. Additionally, ceramics are not prone to postprocessing modifications due to their intrinsic fragility.
One approach that has showed a very promising result is bioceramization, pursued by Tampieri et al. [6]. In more detail, natural wood displays a microarchitecture similar to that of compact bone, constituted by tiny canals and a compact outer shell. Such a bioceramization process encompasses pyrolysis, carburization, oxidation, carbonatation, and finally phosphatization. The final product maintains the complex microarchitecture of natural wood, but is entirely constituted by hydroxyapatite, which is close to the natural mineral phase found in bones. Besides the good mechanical properties of the materials, the scaffolds produced showed excellent properties in terms of osteointegration and bone regeneration.
Taken together, these results open up new scenarios in which tissue engineers can design and fabricate functional scaffolds with tailor-made properties ranging from local bioactivity up to macroscopic mechanical response. This, however, calls for an integrated designing concept in which material properties, fictionalization strategies, fabrication, and postprocessing techniques must be harmonically chosen to meet the stringent and complex requirements of in vivo applications.
In Vitro and In Vivo Studies Toward a Functional Tissue Engineering
The advent of tissue-engineered scaffolds, with their exotic materials and complex shapes, literally revolutionized the world of cell biology whose knowledge and expertise were predominantly based on two-dimensional cell cultures on plastic dishes. The direct implementation of the classic culturing conditions on TE scaffolds highlighted a series of drawbacks like seeding efficacy, cell survival, and phenotypic switches. Therefore, novel culturing procedures had to be specifically developed for TE applications. Additionally, when dealing with in vivo implantations, biologists and clinicians had to find solutions to previously unseen problems. What would cell survival be within the scaffold? What engraftment efficiency can be achieved? How can we modulate interactions between the scaffold and the host dynamically? In this new field, Italian scientists were very active and, in fact, they produced great contributions in regenerative medicine, contributions that are today considered cornerstones in TE.
In general, however, the molecular microenvironment that cell-seeded scaffolds reside in might trigger signaling pathways that force the implant to evolve toward fates that are difficult to predict from in vitro studies only. Along this line, studies aimed at retrieving and analyzing TE implants are of paramount importance since they provide elements to optimize scaffold design along with its treatment before implantation.
Cell colonization and tissue production are not the only aspects that deserve attention when dealing with implanted scaffolds, as proper tissue regeneration should be accompanied by a recovery in functionality of the target tissue or organ. Measuring general functionality, however, is not an easy task since several aspects contribute to the final outcome of an implant. For instance, morphologic, histologic, molecular, biologic, and mechanical aspects must be monitored and assessed to draw out definitive conclusions on the safety and performances of an implant. Diverse groups are currently involved in developing preclinical models and investigative procedures to collect reliable data for implant functionality assessment. Related pilot studies and clinical trials are also providing promising results and valuable feedback for the design and development of more effective and safer scaffolds for a large-scale clinical implementation.
References
- E. Battista, F. Causa, V. Lettera, V. Panzetta, D. Guarnieri, S. Fusco, F. Gentile and P. A. Netti, “Ligand engagement on material surfaces is discriminated by cell mechanosensoring,” Biomater., vol. 45, pp. 72–80, Mar. 2015.
- E. Sandrini, C. Morris, R. Chiesa, A. Cigada and M. Santin, “In vitro assessment of the osteointegrative potential of a novel multiphase anodic spark deposition coating for orthopaedic and dental implants,” J. Biomed. Mater. Res. Part B: Appl. Biomater., vol. 73, no. 2, pp. 392–399, 2005.
- B. A. Harley, A. Z. Hastings, I. V. Yannas and A. Sannino, “Fabricating tubular scaffolds with a radial pore size gradient by a spinning technique,” Biomater., vol. 27, no. 6, pp. 866–874, 2006.
- V. Guarino, M. Alvarez-Perez, V. Cirillo and L. Ambrosio, “hMSC interaction with PCL and PCL/gelatin platforms: A comparative study on films and electrospun membranes,” J. Bioact. Comp. Poly., vol. 26, no. 2, pp. 144–160, 2011.
- C. Palmiero, G. Imparato, F. Urciuolo and P. A. Netti, “Engineered dermal equivalent tissue in vitro by assembly of microtissue precursors,” Acta Biomater., vol. 6, no. 7, pp. 2548–2553, 2010.
- A. Tampieri, S. Sprio, A. Ruffini, G. Celotti, I. G. Lesci and N. Roveri, “From wood to bone: Multi-step process to convert wood hierarchical structures into biomimetic hydroxyapatite scaffolds for bone tissue engineering,” J. Mater. Chem., vol. 19, pp. 4973–4980, 2009.