The group of diseases characterized by the uncontrolled growth and spread of abnormal cells in the body is what defines cancer, and the number of individuals affected each year continues to climb. In the United States, for example, the American Cancer Society reported that men have slightly less than a one-in-two chance of developing cancer sometime in their lives, while women have a little more than a one-in-three risk of developing cancer. These figures for 2013 alone translate to an estimated 1,660,290 new cancer cases, resulting in approximately 580,350 deaths—almost 1,600 people per day, or one every 54 seconds. With an estimated world population of 7.119 billion individuals, of which the U.S. population represents only 4.45%, one can easily realize the importance of finding new or improved treatment modalities for cancer.
All cancers are presently treated with surgery, radiation therapy (also known as radiotherapy), chemotherapy, hormone therapy, biological therapy, and targeted therapy. Except for surgery and radiotherapy, which are local therapies, systemic circulation throughout the vascular network many times remains the only available route to deliver therapeutics. Such unnecessary systemic exposure causes more toxicity for the patient, which often leads to severe side effects, due to the fact that the therapeutic agents accumulate in lower concentrations at the tumor site while also being distributed over healthy tissues and organs.
Passive or active targeting has been and still is being used to increase the therapeutic index, also known as the therapeutic ratio, which represents the amount of therapeutic agents causing therapeutic effects relative to the amount responsible for the toxicity. Although the compromised vasculature is exploited in passive targeting to accumulate therapeutics in pathological sites, active targeting is based on the attachment of specific ligands to the surface of pharmaceutical carriers to recognize and bind pathological cells. However, for both strategies, the agents still circulate systematically prior to the suboptimal accumulation at the site to be treated.
Considering that many of the most deadly cancers are localized, being able to deliver therapeutics directly from the injection site to the targeted region while actively avoiding, or at least reducing, systemic circulation through navigation of pharmaceutical carriers along the most direct route would appear to be a logical approach. Such new paradigms—dubbed direct targeting—pioneered at the NanoRobotics Laboratory, Polytechnique Montréal, could typically be combined with passive or active targeting to further enhance the therapeutic index. Even when metastasis occurs because the cancer is not treated soon enough, several types of cancer can still be targeted directly at another specific region in the body. Indeed, it is difficult for cancer cells to survive outside their region of origin, so they must accumulate and grow in another location with similar characteristics. For example, colon cancer has a tendency to metastasize to the liver, while stomach cancer often metastasizes to the ovary
in women.
But other types of cancer must be treated before metastasis occurs if direct targeting can be considered as a potential efficient treatment modality. Prostate cancer is one example of this because cancer cells usually metastasize to the bones throughout the body. In other cases, direct targeting could be combined with other treatment modalities to achieve better therapeutic effects. This is the case for breast cancer, which metastasizes to the bones, the liver, and the brain—where only the last two locations are potential candidates for direct targeting. Although many types of cancer can benefit from direct targeting after and even before metastasis occurs, direct targeting is not generally appropriate for nonlocalized cancers such as bone, skin melanoma, and blood-related varieties such as leukemia, lymphoma, and myeloma.
Nonetheless, although direct targeting cannot be efficiently used for all types of cancer, it has the potential to enhance significantly the therapeutic index and outcomes for many of the most deadly cancers, and as such, efforts are presently underway to make direct targeting clinically accessible.
The Tumor Environment
At present, direct targeting is considered primarily for delivering therapeutic, diagnostic, imaging, or radioactive agents to solid tumors. Therefore, since direct targeting is a form of medical robotics where algorithms and methods are presently used mainly to control or influence the displacements of therapeutic agents along a planned path, a fundamental knowledge of the tumoral environment where such agents must navigate becomes essential.
The target, a growing tumor, requires access to oxygen and nutrients. When the tumor reaches an overall size of approximately 1 mm, diffusion alone is insufficient for providing these necessities, since the surface area to volume ratio becomes too low. At this point in the development process, the tumor begins to starve. In response, cancer cells send out signals [such as the primary angiogenic growth factor known as the vascular endothelial growth factor (VEGF)] to the cells of nearby blood vessels, instructing them to grow extensions to form supply channels that initiate the formation of a complex and chaotic capillary network known as the angiogenesis network.
But with this additional supply of oxygen and nutrients, hypoxic environments in the solid tumor often result from the rapid proliferation of tumor cells consuming oxygen, which leads to highly heterogeneous oxygen concentrations within the tumor, with approximately <0.7% oxygen [< 5 mmHg partial pressure of O2 (pO2)]. Such a lack of oxygen in deeper regions in the tumor leads to a negative impact on treatments based on radiotherapy, while chemotherapy does not generally affect hypoxic cells that are distant from the blood vessels.
Indeed, compression of the blood vessels by proliferating cells and an inadequate lymphatic system that limits the drainage of excess fluid both contribute to increase the tumor interstitial fluid pressure (TIFP) beyond what is observed in normal tissues. This fact contributes to the further reduction of the therapeutic effect, since the relatively small portion of the drug reaching the vicinities of the region to be treated cannot be diffused deeper in the tumor due to a lack of sufficient flow caused by the TIFP.
Getting There
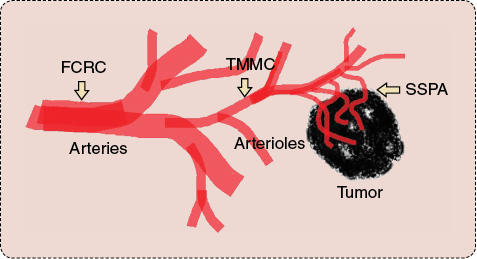
Navigating and delivering therapeutics at the right locations deep inside solid tumors while avoiding systemic circulation are not trivial tasks, when considering the physiological and technological constraints. Because of various physiological features with various environmental conditions along the way as well as the technological limitations, direct targeting typically relies—depending on the type of interventions to be conducted—on a maximum of three main types of carriers to deliver therapeutics deep inside a targeted tumor (similar to launching a spacecraft that requires several modules to reach a remote planet because of technical constraints and the different environmental conditions encountered during travel). As depicted in Figure 1, these three main carriers are a special catheter, referred to as the flow control release catheter (FCRC), for computer-controlled intra-arterial (IA) injections and blood flow modulation; therapeutic magnetic microcarriers (TMMC), for traveling from the release site in larger arteries down to the narrower arterioles; and steerable self-propelled agents (SSPAs) also known as SSP-entities, for transiting in smaller vessels such as the capillaries, the angiogenesis network, and the interstitial space in solid tumors.
Steerable Self-Propelled Agents
This type of agent can be used alone when peritumoral (PT) injections are possible, or it can be combined with the other carriers mentioned in the previous section in the case of IA injections. Ideally, such navigable therapeutic agents must be capable of following the same route as the oxygen and nutrients through the angiogenesis network, since the TIFP prevents direct injections to the tumor. This means that the maximum diameter of each agent is limited to a couple of micrometers because the capillary vessels are just a few micrometers wide (e.g., approximately 4–8 µm compared to approximately 60–100 µm for the thickness of a human hair).
Some level of autonomy embedded in each agent is also required to navigate through the chaotic angiogenesis network. Without medical imaging modalities capable of imaging such tiny vessels for gathering the required information, navigation control from an external source to direct each agent around physiological obstacles is not technologically possible at the present time. Each agent must also have a propelling system that can perform effectively in low Reynolds hydrodynamic conditions (the Reynolds number being in the order of 10−4) such as those encountered when operating in the tumoral environment. The same propelling system, when carrying therapeutic cargo, must also provide sufficient force to transit effectively through the angiogenesis network and to propel each agent deep inside the tumor interstitial space, well beyond the limit of conventional drugs that cannot generally diffuse deeper due to the TIFP. Each agent must also have some sort of steering system to allow an external platform to indicate to each agent the general direction of the tumor.
Furthermore, once past the blood vessels at the entrance of the interstitial space of the tumor, since hypoxic regions cannot be visualized, each agent must have at least one onboard oxygen sensor capable not only of detecting oxygen gradients (decreasing oxygen concentrations from the ends of the blood vessels to the hypoxic regions) but also of influencing the direction of displacement of the agent toward the lower concentrations of oxygen in the tumor interstitial environment. In addition, the same sensor must be able to detect the appropriate lower oxygen concentration of the hypoxic regions and to instruct the agent to maintain its position at the targeted site until the therapeutics are released.
Each agent must also meet both the cytotoxicity and immune system response requirements for potential use in humans. Finally, each agent must have sufficient power to keep all of these embedded functionalities operational for the period of time required for targeting, without relying on known power technologies, which cannot be implemented at such a small scale.
Since such specifications describe a futuristic medical microrobot that is far beyond what could be implemented artificially using modern technologies, one proven strategy considered by our group has been to identify a microorganism that provides all of the above characteristics and functionalities and then to harness the microentity to act as a biological microrobot or microcarrier for drug delivery deep inside solid tumors.
Magnetotactic Bacteria as Therapeutic Microcarriers
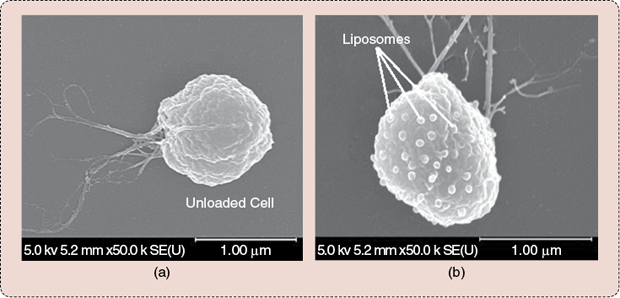
The magnetotactic bacterium of strain MC-1 depicted in Figure 2 has been identified as a potential candidate to fulfill this role. The MC-1 cell is round, with a diameter ranging from approximately 1 to 2 µm. The propelling force provided by two bundles of flagella (flagellar structures are known to be efficient designs to operate under low Reynolds hydrodynamic conditions) connected to rotary molecular motors (with rotors turning 360° inside stators—a design similar to modern engineered motors) allows each cell to swim at an initial average velocity of ~200 µm s−1, with peak velocities reaching ~300 µm s−1 (measured experimentally in phosphate buffered saline at room temperature). The embedded steering system takes the form of a chain of iron-oxide magnetic nanoparticles (MNPs) (~70 nm in diameter) that are synthesized in the cell during cultivation. By inducing a directional torque from a weak magnetic field (typically slightly higher than the geomagnetic field for adequate directional responses from the cell in the microvasculature) on such a chain of MNPs (similar to orientating a magnetic compass needle), magnetotaxis directional control can be implemented. Magnetotaxis directional control entails the cell migrating toward a magnetic pole that is artificially set within the tumor using a special platform dubbed the magnetotaxis system.
Initial analysis suggests that the MC-1 cells do not carry toxic genes, and preliminary tests performed in mice, including cytotoxicity and immune system responses, confirm the fact that they could potentially be used for drug delivery applications in humans. (Tests in primates are scheduled.) Furthermore, this species of bacteria has been successfully cultivated in our laboratory with highly repetitive and reliable propelling and magnetotactic behaviors. This fact makes the MC-1 cell a serious candidate for the production of bacterial-based therapeutics, with highly predictable behaviors for control and targeting purposes.
Besides the fact that these types of sophisticated carriers do not require an additional source of power, the microaerophilic response of the MC-1 bacteria when magnetotaxis directional control is not applied (i.e., when the directional magnetic field is not sufficient to induce a directional torque on the chain of MNPs) causes them to seek ~0.5% oxygen level concentrations, which generally corresponds to the oxygen level expected at the hypoxic regions of solid tumors. With the simplest procedure (since more advanced magnetotaxis control sequences can be applied), an average targeting of more than half in hypoxic regions of tumor xenografts following PT injections has already been achieved in mice. The general sequence of operations to allow this particular SSPA to target and deliver therapeutics in the hypoxic regions of a solid tumor is briefly illustrated in Figure 3.
![FIGURE 3: Targeting and delivering therapeutics to the hypoxic regions of a solid tumor is possible with the MC-1 bacteria. A special platform, the magnetotaxis system, generates an artificial pole within a three-dimensional (3-D) volume (aggregation zone) at the region to be treated. A directional magnetic field [green arrows in (a)] induces a directional torque on the chain of MNPs embedded in the bacteria that causes them to swim toward the tumor. As depicted schematically in (b), adjusting the aggregation zone appropriately where magnetotaxis is replaced by aerotaxis, entails the bacteria to swim past the diffusion limit of conventional drug molecules and to seek the hypoxic zones by swimming through the tumor interstitial space while being guided by a gradient of decreasing oxygen concentration before releasing the therapeutics in hypoxic regions characterized by approximately 0.5% oxygen.](https://www.embs.org/wp-content/uploads/2014/05/37145.png)
Injecting Deeper in the Arterial Network
Starting with the injection site, chemotherapeutic agents can be injected very close to the tumor, such as for PT injections, or further away, such as for intravenous (IV) injections. PT injections can be an option for specific types of cancers, such as colon and prostate cancer, that are more accessible, but therapeutic agents in conventional chemotherapies are typically injected intravenously using various vascular access devices such as infusion devices and various types of catheters.
For direct targeting where PT injections are not an option, IA injections are typically performed instead of IV injections, since arteries carry oxygenated blood to various parts of the body including the tumoral site, and, as such, the blood flow originating from an artery can be exploited to move the navigable therapeutic agents more efficiently toward a tumor.
Prior to placing the catheter used to inject the therapeutics, a guidewire is first introduced in the selected artery. To reach a deeper location in narrower vessels, the guidewire must be thinner, and it becomes much more flexible. Although higher flexibility prevents any risks of perforating a vessel wall, it becomes problematic when maneuvering in narrow and often tortuous arterial vessels due to the increasing friction force between the guidewire and the vessel walls as it goes deeper. At such a stage, the guidewire cannot be only pushed from outside the body; the distal tip must also be pulled to reach deeper regions while preventing problems such as looping. The pulling force must also be directional to allow navigation of the distal tip deeper in the arterial network.
This suggests the use of a strong directional magnetic gradient acting on a magnetic distal tip. Directional gradients in existing magnetic navigation systems (MNSs) dedicated to magnetic catheterization are generated from moving permanent magnets or water-cooled electrical resistive coils located outside the patient. To go far beyond the upper gradient limit of these systems to allow the use of a thinner magnetic guidewire, a superconductive magnet must be envisioned. In a clinical setting, the fringe magnetic field outside a magnetic resonance imaging (MRI) scanner and generated by its superconducting magnet can provide gradients for whole-body interventions that are at least three times stronger than the ones generated by the most powerful MNSs.
However, such a magnet is too bulky to be moved, and the superconductive magnet technology due to the very large inductance of the electromagnet imposes severe constraints, preventing it from being used in a conventional MNS configuration since sufficiently fast variations of the electrical currents required for generating directional gradients are not possible. Instead, directional gradients are achieved by robotically moving the patient slowly within small angles within the fringe field. This is the fundamental idea of another approach pioneered at the NanoRobotics Laboratory that is referred to as fringe field navigation (FFN). Once the magnetic distal tip of the guidewire is positioned as far as possible in the arterial network, the release catheter is then introduced with the guidewire in the catheter lumen. Once the catheter is positioned, the guidewire is then retrieved prior to injecting the navigable therapeutic microcarriers.
Therapeutic Magnetic Microcarriers
Ideally, the distal tip of the release catheter should be able to reach the region to be treated, since it would guarantee that all the drugs are delivered to the treatment site without the risk of increasing toxicity, with a relatively large portion of the drugs bifurcating in untargeted branches at vessel bifurcations during transit. But for reaching a solid tumor, complex vascular networks made of blood vessels much thinner than the instruments themselves, such as arterioles and capillaries, must be transited, which is not technologically possible even when supported with FFN. At best, the distal tip of the release catheter can only be positioned deep in narrower arteries or, perhaps, with further technological advances and depending upon the number of bends and bifurcations along the path adding to the friction force, in larger arterioles at the most. Hence, to eliminate or at least minimize such friction force to allow navigation in narrower vessels, untethered magnetic therapeutic carriers small enough to navigate in such vasculatures have been investigated by our research group for many years.
The MC-1 bacteria cannot typically be injected directly in the arterial network for two main reasons. First, although efficient in the microvasculature, the flagellar propulsion will not be as efficient in larger vessels, and the larger blood flow rate will contribute to sending most of the bacteria into the systemic circulation. Second, even if the blood flow is reduced sufficiently, the distance to be traveled by the bacteria will likely be too long to achieve efficient targeting. Indeed, since the MC-1 cell is nonpathogenic, previous observations indicate that the MC-1 bacteria gradually lose their motility in the first ~40 minutes when exposed to the vascular environmental conditions—a period which is short enough to avoid duplication of the cells in the body. As such, the drug-loaded bacteria must be encased alive in special microcarriers referred to as TMMCs to transit faster and more efficiently from the distal tip of the release catheter to the entrance of the microvasculature, where bacteria are much more effective for targeting purposes.
![FIGURE 4: Of the many possible TMMCs, two examples that can be navigated in the vasculature are shown. (a)–(c) shows a TMMC that was successfully navigated in the hepatic artery to perform liver chemoembolization in preselected targeted lobes in the liver in rabbit models: (a) the MNP, (b) the TMMC, and (c) the general concept. (d) shows another navigable TMMC under development for carrying drug-loaded MC-1 bacterial complexes closer to the microvasculature. (Figure used with permission from [1].)](https://www.embs.org/wp-content/uploads/2014/05/35970.png)
The TMMCs can take various forms, including the two examples depicted in Figure 4, while other configurations are also possible and may be better suited for particular applications and physiological environments. Another type of TMMC not shown in Figure 4 and still under investigation has already shown its capability to temporarily open (a reversible process) the blood–brain barrier, suggesting the possibility of delivering larger therapeutic molecules to the brain.
Magnetic Resonance Navigation of TMMCs Assisted with a Flow Control Release Catheter
The use of permanent magnets to navigate TMMCs in the vasculature is not possible, since the embedded MNPs can only be synthesized with soft magnetic materials. The level of magnetization of such MNPs will then increase only when immersed in a magnetic field. By sufficiently increasing the magnitude or strength of the magnetic field [also referred to as the magnetic field strength (MFS)], the magnetization of the MNPs reaches a maximum level, known as the saturation magnetization. At this saturation magnetization, such MNPs synthesized with the appropriate materials become much stronger magnets (at this scale) than any existing permanent magnets. But the problem with using an external magnet, including an electromagnet, to attract MNP-based TMMCs is that the magnetic field decays extremely rapidly from the external magnet such that when operating slightly deeper in the body, the MFS becomes too weak to be able to influence the path of the TMMCs appropriately.
But it is interesting to note that the uniform field provided by the superconducting technology in the tunnel of a clinical MRI scanner is sufficient to bring the MNPs to, or very close to, the saturation magnetization level. These MNPs are the best candidates for inducing displacement forces through 3-D directional variations of the magnetic field (magnetic gradients), which can be generated by the scanner’s imaging coils being superposed on a uniform field. The use of the MRI scanner to provide an appropriate environment for the effective navigation of therapeutics in the blood vessels is the fundamental idea of another approach developed at the NanoRobotics Laboratory and known as magnetic resonance navigation (MRN). (See Figure 5.) MRN has been successfully used in the hepatic artery of live rabbits for the TMMCs depicted in Figure 4(a)–(c) with controlled release, following our previous demonstration of the first untethered object navigated in the blood vessels, with the experiment performed in the carotid artery of a live pig.
![FIGURE 5: Some examples related to MRN. (a) MR-image artifact created by a 1.5-mm chrome-steel bead in the carotid artery of a pig. (b) Image of the first in vivo MRN where a 1.5 mm-chrome-steel bead in the carotid artery of a pig. The MR-artifact was used with a specially developed MR-tracking algorithm known as MS-SET to gather the positional data for feedback navigation control being performed at 24 Hz. The bead was navigated at an average velocity of 10 cm/s. The position was plotted automatically using an icon superposed on previously taken MR and X-ray images. (c) Image of blood vessels from a live rabbit obtained with a 1.5 T clinical MRI scanner. In the image, an icon is superposed to indicate the position of the magnetic object. (d) MR-image of a few TMMCs at a specific targeted location within the liver of a rabbit following MRN in the hepatic artery. (e) MR-image of a vessel not visible using X-ray modality. The MR-artifacts created by the magnetic agents were used to visualize such vessels with a diameter below 200 µm, i.e., beyond the spatial resolution of any existing medical imaging modalities. (Figure used with permission from [2] and [3].)](https://www.embs.org/wp-content/uploads/2014/05/37180.png)
To be more effective, the computed directional gradient fields are used to keep the TMMCs along a planned trajectory in the arterial network and the arterioles, while the blood flow is used for propulsion purposes. Because of the high flow rate combined with the time required to perform magnetic resonance tracking of the TMMCs to gather positional data for computing the required corrective actions to navigate them along the trajectory, a special catheter, the FCRC, is being considered. The FCRC is a computer-controlled apparatus that is synchronized with the navigation sequences and accordingly modulates the blood flow in an optimal manner, ensuring maximum targeting efficacy within the various technological and physiological constraints. It also releases aggregations of TMMCs with specific sizes, depending on various parameters and physiological conditions.
The fundamental engineering presented here has already been validated in animal models. More work involving medical specialists is presently underway to develop appropriate interventional protocols as well as new navigable therapeutics. Additional data are being gathered to confirm safety issues. Nonetheless, the recent results suggest that this new paradigm may be considered for preliminary human trials in the near future.
To Probe Further
Readers will also find many scientific papers on various specialized aspects related to these technologies that have not been described here. Some more general descriptions of these technologies are also available on the Internet. What follows is a list of some examples.
- Fighting cancer with nanotechnology—TEDx presentation in French with subtitles in English. [Online].
- AZoNano.com. Magnetic resonance navigation of nanorobotic cancer therapies: an interview with Professor Sylvain Martel. [Online].
- Harnessing nanotechnology in the fight against cancer. The Globe and Mail. [Online].
- Magnetic microbots to fight cancer. IEEE Spectrum. [Online].
- Video showing an example of aggregation of the MC-1 bacteria under control: Swarm of bacteria build tiny pyramid. [Online].
- Voyage of the Bacteria Bots. MIT Technology Review. [Online].
- Bacteria take the fantastic voyage. NewScientist. [Online].
- MRI scanner steers magnetic particle in live animal’s blood. NewScientist. [Online].
Acknowledgments
The author would like to thank several funding organizations for their support and vision by providing the resources required to pursue the development of these technologies. The major players include Polytechnique Montréal, Univalor, Consortium Québécois sur la Découverte du Médicaments, the Canada Research Chair Program, the Natural Sciences and Engineering Research Council of Canada, the Government of Québec, Canadian Funds for Innovation, the National Institutes of Health, and several other organizations that contributed financially to our partners and students.
The active participation of many individuals besides the staff, graduate students, and technicians is worth mentioning, and the list of participants in such highly interdisciplinary projects to make it accessible clinically in the future is growing.
In alphabetical order (current immediate collaborators only):
- G. Batist—oncologist, McGill University
- N. Beauchemin—biochemist, McGill University
- G. Beaudoin—medical physics and MRI sequencing, University of Montréal
- F. Cheriet—medical imaging, Polytechnique Montréal
- L. Gaboury—pathologist, University of Montréal
- S. Kadoury—medical image registration, Polytechnique Montréal
- M. Lafleur—chemist, University of Montréal
- M. Mohammadi—biologist, bacterial culture, Polytechnique Montréal
- D. Radzioch—immunologist, McGill University
- G. Soulez—interventional radiologist, University of Montréal
- M. Tabrizian—biomaterials and biointerfaces, McGill University
- T. Vuong—radio-oncologist, McGill University.
References
- P. Pouponneau, J.-C. Leroux, G. Soulez, L. Gaboury, and S. Martel, “Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation,” Biomaterials, vol. 32, no. 13, pp. 3481–3486, May 2011.
- S. Martel, “Magnetic therapeutic delivery using navigable agents,” Therapeutic Delivery, vol. 5, pp. 189–204, Feb. 2014.
- S. Martel, “Navigation control of micro-agents in the vascular network: challenges and strategies for endovascular magnetic navigation control of microscale drug delivery carriers,” IEEE Control Systems, vol. 33, no. 6, pp. 119–134, Dec. 2013.