As antibiotic resistance rises, the promise of bacteriophages offers treatment options
Bacteriophages once were a promising prospect to treat bacterial infections but fell out of favor by the mid-20th century with the advent of highly effective antibiotics. As antibiotic resistance has spiked in recent years, however, interest in phages has risen, particularly following a number of last-resort phage treatments that were able to beat severe antibiotic-resistant strains. New research is now beginning to show that phage therapy may become much more broadly used as treatments for diseases and other infections.
Mixing a broad TB cocktail
A University of Pittsburgh lab has spent more than three decades studying bacteriophages—everything from life cycles and evolution to genomics, gene function, and gene regulation—and since 2017, has ventured onto the clinical side. Their first effort involved engineering a three-phage concoction to treat a dangerous Mycobacterium abscessus infection in a teenaged patient with cystic fibrosis [1]. “That was the first use of bacteriophages to treat a Mycobacterium infection, and it was a one-off thing for compassionate use,” described lab leader Graham Hatfull, Ph.D., Eberly family professor of biotechnology (Figure 1).

Figure 1. Graham Hatfull, University of Pittsburgh Eberly family professor of biotechnology, and his research group are working on a phage cocktail that is broadly effective against Mycobacterium tuberculosis, the bacterium that causes tuberculosis. Most of his decades of research on bacteriophages has been on the basic-research side, but over the past five years, he has helped develop personalized bacteriophage treatments for patients with antibiotic-resistant strains of Mycobacterium species. Hatfull is shown here holding a vial of a therapeutic phage sample. (Photo courtesy of Aimee Obidzinski/University of Pittsburgh.)
Since then, the lab has been busy with a series of compassionate-use cases for individual clinical isolates of Mycobacterium species (e.g., [2], [3]), while also continuing to study the fundamentals, including why bacteriophage therapies for M. abscessus must be personalized, but those for M. tuberculosis need not, Hatfull said. “This gets into an area in which we’re intensely interested and, from a biological point of view, is the thing in which I think everybody should be interested: What determines how and why some phages infect certain bacteria or certain strains, but not others?” He and his lab have narrowed down the answer, at least in part, to prophages. “M. abscessus carries so-called prophages, or phage sequences that are integrated into its chromosome and/or plasmids in a diverse way, and M. tuberculosis doesn’t. In fact, plasmids and prophages are pretty much absent from M. tuberculosis,” he explained. “That, we think, is playing an important role in determining how viruses infect those bacteria.”
To learn more about phage-infection sensitivity profiles, Hatfull and his group are taking a rather experimental approach. “Prophages are sort of silent and hiding away inside the genomes of these M. abscessus strains, but we’ve identified ways of essentially waking them up, teasing them out, and then actually growing them in the lab,” he said. The prophages could be therapeutically useful themselves, but beyond that, his lab is inserting them into prophage-free strains and watching what happens. “If a prophage does make a difference—and we have some preliminary data that’s says that’s true—then we can clone the genes, do transcriptomics to find which genes are expressed, and essentially strip it right down to the molecular details to find out how that works,” he said.
Such experimentation will provide a better understanding of the defensive tactics bacteria use against phages, such as CRISPR CAS systems and restriction enzymes, and the countermeasures phages use to get past them. “This is how we’re going to learn to control the specificity, and how we can go from having very tight, personalized medicine to building bacteriophages that work much more broadly,” Hatfull said.
He and his group have developed a five-phage cocktail and tested it in the lab to determine whether it has broad effectiveness against M. tuberculosis [4]. They compared the one particular phage strain (called Muddy) against a set of reference strains of M. tuberculosis, and found that it infects about half of the strains but not the other half. They then isolated a mutant—a variant—that was capable of infecting a strain in the other half. “When we characterized that, we found we could expand the host range, so the phage could then infect all of the reference strains of M. tuberculosis,” Hatfull recalled. “Experimentation combined with phage screening, some engineering, and genetics allowed us to identify a core set of phages—this modest cocktail—that acts against tuberculosis broadly, rather than having to personalize it for each individual strain” (Figures 2 and 3).

Figure 2. Clear circles on this bacteria-coated plate show where phages have performed well. Hatfull and his group hope to continue doing basic research to understand phage biology, while also learning from one-off phage treatments for patients who have especially antibiotic-resistant infections. (Photo courtesy of Aimee Obidzinski/University of Pittsburgh.)
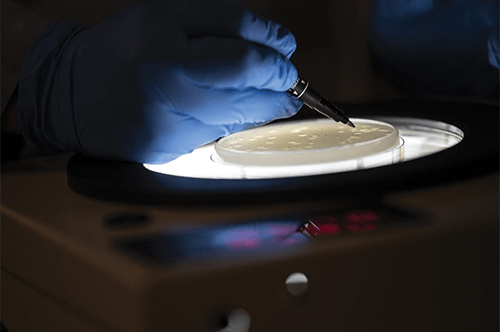
Figure 3. With careful inspection, Hatfull is able to isolate a candidate phage from the plate. Through work like this, he and his group have developed a five-phage cocktail and obtained good results against M. tuberculosis in a lab setting. (Photo courtesy of Aimee Obidzinski/University of Pittsburgh.)
He added, “The opportunity is this: An understanding of all that biology allows you to learn to control the specificity, and that is how you go from having very tight, personalized medicine, as you have with phages for M. abscessus, to being able to design something to act broadly. That is where we’d like to be if we can.”
For epidemic cholera
In Boston, MA, USA, researchers are zeroing in on cholera as a target for broadly effective phage prophylaxis. Minmin “Mimi” Yen, Ph.D. (Figure 4) began investigating phages to fight epidemic cholera about a decade ago while she was a doctoral student in the research group of Andrew Camilli, Ph.D., professor of molecular biology and microbiology at Tufts University, and the two have since co-founded the spinoff company PhagePro (Yen is chief executive officer, Camilli is scientific advisor) to move toward future commercialization.
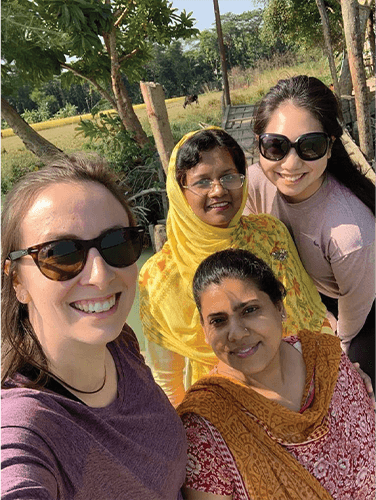
Figure 4. Minmin “Mimi” Yen (right rear), CEO and co-founder of PhagePro, has been collaborating with the International Centre for Diarrhoeal Disease Research in Bangladesh (abbreviated icddr,b) in the hopes of bringing a phage treatment, called PVC, to the people of that country and other resource-limited regions of the world as a way to battle cholera. Pictured with Yen are icddr,b scientist Marzia Sultana, Ph.D. (in yellow), scientist Fatema-Tuz Johura (orange scarf), and former PhagePro principal scientist Gabrielle Grandchamp, Ph.D. (Photo courtesy of icddr,b collaborators.)
Cholera was a particularly good candidate for broadly acting phage prophylaxis because only a few strains of the offending bacterium—Vibrio cholerae—are behind nearly all of the disease outbreaks, Yen said. “An outbreak in Bangladesh most likely has the same strains that would cause outbreaks in Africa, so we were interested in developing an almost one-size-fits-all phage cocktail that could be rapidly distributed all around the world regardless of whether it’s an endemic situation where the country experiences outbreaks periodically, or it’s during a human rights violation and in a refugee camp.”
Contributions from postdoctoral researchers Lynne Cairns and Kimberley Seed (now assistant professor at the University of California, Berkeley, CA, USA), along with others in the Tufts Lab helped the project accelerate quickly, and they soon had a phage cocktail that could prevent cholera symptoms in animal models [5].
In 2016 while the lab investigation was continuing, and Yen and Camilli were concurrently starting up PhagePro, Yen went back to school (this time at Boston University) to pursue a master’s in public health as a way to grasp the complexities of creating technology for the resource-limited settings where they hoped to one day put their work into practice. “I wanted to make sure that the focus was always on the communities, so we had to incorporate that really early on in the research process,” she explained, noting that she also spent time in Haiti to understand health care limitations and possibilities. A community focus helped steer the researchers toward a phage delivery method that was orally administered, stable in hot and humid conditions, required no refrigeration, and was able to be self-administered.
So far, the researchers have shown proof-of-concept for a liquid formulation of a cholera phage cocktail called ProphaLytic-Vc (PVC) (Figure 5), and with a recent US $3 million National Institutes of Health Grant [6], have begun testing it against clinical isolates obtained via the International Centre for Diarrhoeal Disease Research in Bangladesh (abbreviated icddr,b) during outbreaks in that country. They are also investigating how to adapt the phage cocktail so it fights any resistance that may crop up, she said, and ultimately hope to run the first in-human trial at the icddr,b. The trials in Bangladesh will not only provide valuable insights into PVC’s effectiveness against cholera, but also how well the therapy fits into a health care venue in a low-income region, Yen said. “This is a way of rethinking global health partnerships so we have strong tech transfer expertise both ways, and we end up with equitable distribution and capacity building.”

Figure 5. Initial work on PVC (cholera on a plate shown here) began while Yen was a doctoral student in the lab of Tufts University Professor Andrew Camilli, and PhagePro is now optimizing the treatment. PVC is designed as a broad-acting phage therapy against disease-causing Vibrio cholerae, the bacteria behind this acute and sometimes life-threatening diarrheal illness. (Photo courtesy of Minmin Yen.)
Yen believes the phage cocktail meshes with the World Health Organization’s vision of rapid-response teams who are quickly deployed to help with cholera outbreaks. Such teams may offer cholera vaccination, but vaccines need about two weeks to become effective, she said. In contrast, PVC begins working immediately, so it could fill the gap and provide protection until vaccine immunization kicks in. And if the researchers are able to develop a powder version for manufacture into a tablet, she added, PVC could be distributed quickly and easily.
At this point, the researchers are focusing on cholera, but they are keeping an even bigger picture in mind, Yen said. “We hope we can take all the experience and expertise that we are gaining and apply it to additional areas, starting with other enteric diseases, but wherever we take it, we will follow our mission and our emphasis on the global health side and vulnerable communities.”
For wounds
At the University of California Los Angeles (UCLA), a research team is trying something different for antibiotic-resistant wound bacteria. Rather than using phages as the weapons, this multidisciplinary group is using them as transport vehicles for the real agents of destruction: gold nanorods that can reach bacteria-destroying temperatures. “There is a little bit of fine tuning to make sure that you don’t damage the tissue thermally, but there is this window where you can kill the bacteria without really damaging the tissue,” asserted project lead Irene Chen, M.D., Ph.D., associate professor of chemical and biomolecular engineering at the UCLA Samueli School of Engineering (Figure 6).

Figure 6. Irene Chen, associate professor of chemical and biomolecular engineering at the UCLA Samueli School of Engineering, and her group are using phages to kill wound bacteria. In their approach, the phages carry gold nanorods to the bacteria, and with the addition of a bit of laser light, the nanorods introduce just enough heat to kill the bacteria. (Photo courtesy of Rebecca Farmer.)
The idea of using gold-loaded phages originated with postdoctoral researcher Huan Peng (Figure 7), who suggested employing them to detect bacteria. The phages would carry the gold nanoparticles to a destination and as the gold nanospheres aggregated, they would change color and signal an infection. It worked [7].

Figure 7. Chen credits postdoctoral researcher Huan Peng (pictured) and his materials-synthesis background with suggesting the idea of loading phages with gold nanoparticles. He had originally suggested using them to detect bacteria, because an aggregation of gold nanospheres changes color. One thing led to another, and soon the research group began investigating whether they could use the gold-laden phages to find and also eliminate bacteria at a wound site. (Photo courtesy of Irene Chen.)
Before long, the research group began investigating whether they could use the gold-laden phages to not only find, but also eliminate bacteria at a wound site. Chen explained, “Gold nanorods are metals that have a sea of electrons that will resonate coherently together (called surface plasmon resonance), so if you shine them with a laser light at a certain resonant frequency, they absorb that light strongly and then dissipate that energy through vibrations in the gold lattice. Those vibrations become heat that is sufficiently hot to kill the target bacteria” [8].
Chen’s group also tried antibodies as the gold carrier, but found phages did a better job. She isn’t certain why, but thinks it may have to do with delivery. For one thing, a phage is much larger than an antibody and can carry at least 10 times more gold nanoparticles, she said. For another, phages deliver the nanoparticles to a concentrated location on a cell wall, whereas antibodies typically bind in various locations on the cell wall. “My thinking is that even if you use antibodies and phages to deliver the same number of nanoparticles to the bacterial cell, the phages’ concentrated placement of the gold nanorods gives a sharper thermal gradient that is more effective at killing the bacterial cells vs. the more diffuse heating over the entire cell that occurs with antibody-delivered nanoparticles,” she remarked.
For this project, the researchers used the very well-studied M13 Escherichia coli phage, swapping out the binding protein so it latches onto Pseudomonas aeruginosa, the bacterium behind many hospital infections. Through collaborations with chemist Sheref Mansy of the University of Alberta, Canada, and physiologist Kenneth Roos at UCLA, the researchers together employed a combination of nanomaterial synthesis and bioconjugation chemistry to add the gold nanorods, plus a peptide that releases zinc to promote wound healing.
They then compared a wound application of the gold- and zinc-laden phages to a standard antibiotic treatment to determine how well each worked against Pseudomonas aeruginosa in a mouse model [9]. The test results showed that, compared to the antibiotics, the phage nanorod treatment provided a 10 times greater reduction in bacterial load and about three times faster healing.
The phages showed other positive aspects too, Chen said. “We tested bacterial strains that were resistant to polymyxins, which are a last-line antibiotic that physicians use, and we found that the antibiotics didn’t work. But the phage nanorod therapy did work, and that makes sense, because the mechanism of resistance to polymyxins has nothing to do with the ability of the phage to bind.” In addition, the phage nanorod treatment displayed none of the significant side effects, such as renal toxicity, that are associated with polymyxins and a variety of other antibiotics, which suggests that phage nanorods might be a good alternative for patients who have renal issues “and cannot tolerate the additional burden of antibiotics,” she said.
Beyond that, this approach means the phages have no need to infect the host to kill it, she noted. “One of the major issues with phage therapies is that they may have biological effects that we don’t understand,” she said. “For instance, there are some phages that transduce genes, or take genes from one host bacterium and transfer them to a different one, which means that you have potential for a phage transferring genes around in the microbiome. In addition, some phages carry toxic proteins, and some assemble into biofilms that are especially difficult to eradicate, so there are all sorts of unintended consequences that need to be considered.” By using the phages as transportation only, she said, those potential issues fall away.
With the success so far, Chen and her group have begun looking beyond Pseudomonas aeruginosa. They have already developed gold-carrying phages for four additional bacteria, and will be interfacing with clinicians to learn both which target organisms they would like to see added, and how they envision this being integrated into health care practice.
Future for phages
All three researchers see phage therapy once again taking a place in health care arsenal.
Hatfull remarked, “Generally, I suspect it will be rare for phages to ever replace antibiotics—they’re more likely to be used in combination with them as adjuncts, or perhaps to enhance the activity of the antibiotics—but once we understand the basics of phages, including the biology, genomics, gene function and that sort of thing, we will be able to develop strategies that will give you broad application against disease-causing bacteria, just as we’ve been able to do with M. tuberculosis.”
The pieces are starting to fall into place for phage therapy with basic-biology and translational research underway, industry interested, regulatory agencies starting to get behind it, and doctors becoming more open to trying it, Yen said. “A lot of clinical trials are beginning in this field, and once we have the first successful phase 2 clinical trial, that’s going to be the ultimate tipping point.”
Antibiotic resistance will also push the field forward. “We know antibiotic resistance will build over the next several decades, so we need to be prepared,” Chen remarked. “My hope is that, as a community, we’ll have this long and expanded list of solutions, and then, when the time comes to deploy them, we’ll be able to get them out. And I see no fundamental reason why phage therapy in some form can’t be among them. I think it’s really just a matter of engineering them and pushing this field forward.”
References
- R. M. Dedrick et al., “Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus,” Nature Med., vol. 25, no. 5, pp. 730–733, May 2019.
- J. A. Nick et al., “Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection,” Cell, vol. 185, no. 11, pp. 1860–1874, May 2022.
- J. S. Little et al., “Bacteriophage treatment of disseminated cutaneous Mycobacterium chelonae infection,” Nature Commun., vol. 13, no. 1, May 2022, Art. no. 2313, doi: 10.1038/s41467-022-29689-4.
- C. A. Guerrero-Bustamante et al., “Toward a phage cocktail for tuberculosis: Susceptibility and tuberculocidal action of mycobacteriophages against diverse Mycobacterium tuberculosis strains,” mBio, vol. 12, no. 3, Jun. 2021, Art. no. e00973.
- M. Yen, L. S. Cairns, and A. Camilli, “A cocktail of three virulent bacteriophages prevents Vibrio cholerae infection in animal models,” Nature Commun., vol. 8, no. 1, Feb. 2017, Art. no. 14187, doi: 10.1038/ncomms14187.
- U.S. National Institutes of Health. NIH Awards Grants to Support Bacteriophage Therapy Research, Mar. 11, 2021. [Online]. Available: https://www.nih.gov/news-events/news-releases/nih-awards-grants-support-bacteriophage-therapy-research
- H. Peng and I. A. Chen, “Rapid colorimetric detection of bacterial species through the capture of gold nanoparticles by chimeric phages,” ACS Nano, vol. 13, no. 2, pp. 1244–1252, Jan. 2019.
- H. Peng et al., “Controlled phage therapy by photothermal ablation of specific bacterial species using gold nanorods targeted by chimeric phages,” Proc. Nat. Acad. Sci. USA, vol. 117, no. 4,
pp. 1951–1961, Jan. 2020. - H. Peng et al., “Treatment of wound infections in a mouse model using Zn2+-releasing phage bound to gold nanorods,” ACS Nano, vol. 16, no. 3, pp. 4756–4774, Mar. 2022.