We understand now that sleep of sufficient length and quality is required for good health. This is particularly true for infants and children, who have the added physiologic task of growth and development, as compared to their adult counterparts. Sleep-related breathing disorders (SRBDs) are common in childhood and if unrecognized and not treated can result in significant morbidity. For example, children with obstructive sleep apnea (OSA) can exhibit behavioral, mood, and learning difficulties. If left untreated, alterations in the function of the autonomic nervous system and a chronic inflammatory state result, contributing to the risk of heart disease, stroke, glucose intolerance, and hypertension in adulthood.
The most common perturbation of breathing during sleep in childhood is the OSA syndrome, affecting as many as 4% of the pediatric age group. OSA is characterized by repetitive bouts of complete or partial upper-airway obstruction during sleep, resulting in episodes of hypoxemia, hypercapnia, and sleep disruption. The latter is related to arousals from sleep in response to airway occlusion and/or abnormalities of gas exchange. The most common cause of OSA in children is hypertrophy of the tonsillar and adenoidal tissue. This can usually be adequately treated by removal of the offending tissues, and tonsillectomy is the most common pediatric surgery in the United States. However, there are other more complicated medical conditions leading to OSA, and these pose a significant therapeutic challenge to clinicians and surgeons alike.
The upper airway is an exceedingly complicated structure that begins at the nares and terminates at the glottis or vocal cords. It is divided into several zones: the nasal passages, the nasopharynx, the oropharynx, and the hypopharynx. The upper airway is critical not only for breathing but to speech and swallowing, and each of these functions places specific demands on the performance of the upper airway. In addition, these functions must be coordinated—for example, swallowing requires temporary suspension of breathing and closure of the glottis to prevent aspiration. To accomplish these various tasks, the upper airway is equipped with more than 30 pairs of muscles, some with dual innervation by both voluntary and involuntary systems of respiratory control.
The vulnerability of the upper airway to collapse and occlusion is nested in the requirement for a flexible and highly mobile structure to produce speech and to safely swallow food and liquids. A further element of vulnerability is introduced by the changes imposed by sleep. Upper-airway muscle tone falls during sleep, becoming nearly absent during rapid eye movement (REM) sleep, thus predisposing children to airway obstruction even under optimal conditions. Designed from the ground up, the pathway for breathing fresh air into the lungs would not be the complicated system evolution has provided us, but a rigid tube free of risk from collapse during sleep. Knowing these features of the upper airway, imagine the difficulties for children who have narrowing of their pharynx from obesity, were born with an anatomically small airway, or have poor neuromuscular tone due to intrinsic problems with neuromuscular function. These children can have very severe OSA and can be the most difficult to treat. Herein lies the challenge for sleep medicine clinicians and surgeons—how to evaluate this complex structure and accurately plan interventions to restore and maintain airway patency and function.
Documentation of the severity of OSA is fairly straightforward via the use of polysomnography (PSG)—multichannel recordings of electrophysiological, respiratory, and cardiac function performed overnight and evaluated for sleep architecture, the number and types of apneas, and the resulting abnormalities of gas exchange. For children who are not candidates for tonsillectomy, the next line of treatment for OSA is positive airway pressure (PAP), which can be thought of as a “pneumatic splint” holding the airway open during sleep. PAP is delivered by a bedside device, with the patient wearing a snug-fitting mask over the nose or nose and mouth. But many children, especially those with developmental disabilities, are unable to tolerate this treatment, and other treatment options are needed. Surgery to prevent airway collapse is the next logical step, but how much tissue to remove or how extensively to change the position of the facial bones is a conundrum.
This was the dilemma that brought together a unique group of scientists to ponder how the upper airway functions and how the anatomy could be captured in ways that would allow identification of the sites of obstruction to create models that could be virtually manipulated to plan and predict the results of surgical interventions. A four-year process, funded by the U.S. National Institutes of Health and capitalizing on knowledge borrowed from fields as disparate as fuzzy logic, computational fluid dynamics (CFD), and clinical medicine, has brought us closer to solving this problem. The utilization of advanced imaging technologies capturing the dynamics of the upper airway during sleep and wakefulness from populations of children at risk for OSA have informed the physicists, engineers, and mathematicians to refine the application of science borrowed from other fields to potentially create accurate models in both anatomy and function. The advances made by this blended family of scientists will bring new tools and techniques to the clinicians caring for these children. The various approaches each team has adopted to tackle this clinical problem are the focus of the sections that follow (see also “Functional Modeling of the Pediatric Upper Airway”).
[accordion title=”Functional Modeling of the Pediatric Upper Airway”]
By Carol J. Blaisdell, M.D.
The upper airway in children can be the site of multiple types of congenital malformations and acquired syndromes of airway obstruction. Intensive care units for neonates and children care for an increasing number of these children, consuming significant resources (10–20% of neonatal intensive care unit/pediatric intensive care unit beds in tertiary care settings). Individuals with maldevelopment of craniofacial, soft tissue, laryngeal, and tracheal structures of the upper airway account for approximately 1,500 visits/year to the typical academic pediatric otolaryngologist. Clinicians have limited tools for evaluating the consequences of narrowed upper-airway structures and recognize that two-dimensional snapshots of the airway with radiographs, CT scans, and MRI do not provide sufficient information regarding upper-airway function on which to base their decisions. Even endoscopy does not delineate the entire picture when considering how sleep/wake states impact function.
Current treatment of upper-airway disorders in children is limited to bypassing the obstruction in severe cases (tracheostomy, which carries significant risk and long-term morbidity), craniofacial reconstruction (which is difficult to perform without a roadmap for success), or removing tonsils and/or adenoids, leaving at least 20% of children with residual sleep-disordered breathing. As many as 50% of children with congenital craniofacial malformations have obstructed breathing, detected by PSG, but the sites of upper-airway obstruction can be multiple (large tongue, microagnathia, midface hypoplasia, laryngomalacia, to name a few), and PSG does not identify which sites of obstruction are the most important.
The upper airway serves three important functions: conduction of air, swallowing, and speech. To accommodate these functions, its shape is actively modulated by neural input but is passively collapsible at times. During postnatal development, it undergoes significant structural and functional changes that affect its size, shape, and mechanical properties. Another important characteristic of the upper airway is that it is a virtual conduit with anatomical boundaries defined by other tissues of varying compliance that determine instantaneous properties. Abnormalities of the upper airway require prompt attention because these often alter ventilatory patterns and gas exchange, particularly during sleep when upper-airway motor tone and ventilatory drive are diminished, leading to hypoxemia, and can result in respiratory insufficiency/failure. The long-term consequences of these perturbations include cognitive deficits with an attendant lifelong impact on the child.
Clinicians feel limited by the tools they use to evaluate upper-airway structure, recognizing that structure does not often correlate with function, making decisions regarding interventional options problematic. Imaging techniques are advancing rapidly, but haven’t been applied to study what key components of upper-airway structures contribute to dysfunction and under what circumstances. Computational and fluid dynamic models to understand flow limitation of cardiac and vascular structures have not been applied to study upper-airway function with powerful analytical tools using clinically relevant data.
In 2010, the National Heart, Lung, and Blood Institute launched a program, Functional Modeling of the Pediatric Upper Airway (RFA HL10-017). The goal of this initiative was to develop functional models of the upper airway to identify control points that limit airflow. These control points were expected to vary with the respiratory cycle and the underlying etiology of airflow limitation (skeletal malformations, soft tissue hypertrophy, and muscle tone) and often involve multiple tissue components. The ultimate goal in advancing this research was to develop novel approaches for interventions that could more effectively correct upper-airway flow limitation in children.
The Functional Modeling of the Pediatric Upper Airway Program has supported five multidisciplinary research teams (radiologists, bioengineers, mathematicians, clinicians, and scientists) to investigate the upper airway using minimally and/or noninvasive imaging (MRI, ultrasound, and optical coherence tomography) and computational modeling. Each research team has enrolled pediatric subjects and gathered and analyzed imaging data to predict points of upperairway dysfunction during the respiratory cycle. Because the upper airway is a complex structure, models include imaging of soft tissue, skeletal, and neuromuscular components of the upper-airway conduit over time (for example, during sleep, wakefulness, feeding, agitation, and sedation) and have been shared across research centers to further validate preliminary findings. A virtual pediatric airway is being developed as a resource for clinicians and scientists interested in understanding the complexity of this vital structure.
Carol Blaisdell (blaisdellcj@nhlbi.nih.gov) is a medical officer for lung development and pediatrics at the National Heart, Lung, and Blood Institute of the U.S. National Institutes of Health.
[/accordion]
OSA and Down Syndrome
The Cincinnati research team has focused their efforts on a group of children at high risk for OSA, those with Down syndrome. In otherwise healthy children with OSA due to adenotonsillar hypertrophy, surgical removal of the tonsils and adenoids (AT) is considered to be the first line of treatment. While most nonobese children with OSA respond favorably to AT, a large percentage of children with obesity or with abnormal structure and function of the upper airway in association with genetic syndromes such as Down syndrome have persistent airway obstruction during sleep after surgery. Despite the large number of children undergoing different treatments for OSA, the predictors of success of any specific therapy are poorly understood. The structural and functional properties of the upper airway, i.e., shape, size, and compliance, and the integrative central controller of breathing should function in perfect harmony during the respiratory cycle to maintain the patency of the upper airway during sleep. A perturbation of any of these components can destabilize upper-airway control, leading to loss of synchrony between airway patency and phases of the respiratory cycle, potentially leading to a closed airway during inspiration.
To enhance the clinicians’ ability to predict the outcome of specific therapies of OSA in children, Dr. Raouf Amin and colleagues have employed computational modeling and analysis of fluid structure interactions. Their overarching objective is to predict the success of surgical treatment of children who have persistent OSA despite previous AT. This is particularly true of children with Down syndrome, and it is of great interest considering how difficult it is to use mask PAP for these children. Toward that end, the Cincinnati team has developed an approach to characterize the upper-airway anatomy from static magnetic resonance imaging (MRI) with estimations of airway compliance and closing pressure. These data are used to develop a better understanding of the dynamic fluid structure interaction of the upper airway.
As a first step, the modulus of elasticity (a relationship between applied stress and resulting strain representing the resistance of a structure to deformation) is determined from MRI of the upper airway during breathing at different positive pressures. The displacements of the anterior, posterior, and lateral airway walls at the retroglossal and retropalatal regions contribute to the calculation of regional modulus of elasticity.
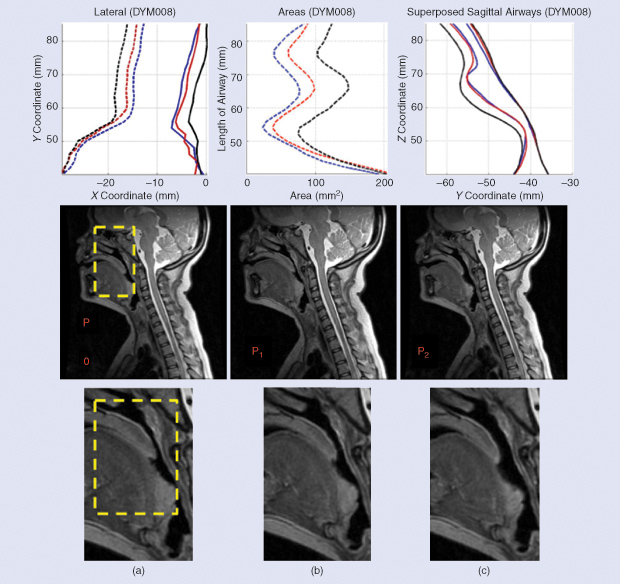
Figure 1 shows the MRI of the upper airway under atmospheric pressure and two PAPs. The diagrams show the displacement of (a) the lateral walls, (b) the change in the cross-sectional area along the length of the airway, and (c) the displacement of the anterior and posterior walls under atmospheric pressure (blue line), low-level positive pressure (red line), and high positive pressure (black line). The modulus of elasticity is then used to develop an individualized dynamic fluid–structure interaction model to predict the response of the upper airway to different levels of closing pressures, as shown in Figure 2.
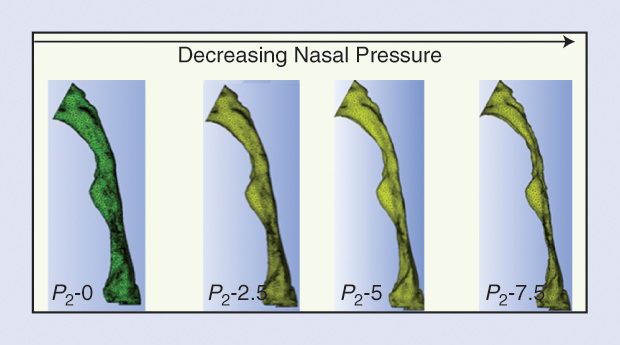
The clinical implications from using a computational approach to OSA are to first determine who is likely to benefit from a medical therapy such as continuous PAP during sleep versus surgical interventions. The delineation of local areas of collapse in the airway can determine the best choice of surgical procedures that may target the soft tissue structures of the upper airway or its bony boundaries. This is of considerable importance in the care of children with Down syndrome, who not only have abnormalities of the soft tissues, such as macroglossia, but also compromised boney anatomy with midface hypoplasia.
Obesity and OSA Syndrome
The group of bioengineers and clinical researchers at the University of Southern California and Children’s Hospital Los Angeles has focused on a common pediatric problem for their study of the upper airway, that of obesity. The prevalence of childhood obesity has increased dramatically over the past three decades. A significant fraction of these obese children also have an SRBD. However, this population exhibits a broad variety of sleep and clinical characteristics that may vary from children who only snore and do not have abnormalities of gas exchange or sleep disruption to traditional OSA syndrome with recurrent episodes of complete and partial airway obstruction.
The research group employs computational modeling and state-of-the-art dynamic MRI (DMRI) of the upper airway along with physiological measurements during sleep to test the hypothesis that the diversity in phenotypic sleep and breathing behavior results from the existence of different underlying physiological mechanisms. They also hope to determine whether a quantitative dynamic model can lead to better delineation of these mechanistic differences.
Obese teens who snore undergo an overnight session in which polysomnographic variables are recorded, and the following experimental interventions are applied:
- baseline (no intervention)—from these measurements, the plant dynamics transfer function is estimated by relating the changes in ventilation to corresponding fluctuations in end-tidal PCO2.
- determination of the ventilatory response to ventilator-induced sighs
- determination of the upper-airway critical closing pressure
- determination of arousal threshold and arousal drive by performing brief airway occlusions (mimicking obstructive apnea) while the subject is sleeping.
The aforementioned protocols are administered throughout the overnight study on a semiautomated basis using an integrated measurement and control system developed by the team.
The estimation of the key parameters from these test procedures provides an alternative means of phenotyping SRBDs that is based more on underlying physiological mechanisms than on polysomnographic measurements. An important outcome of this research has been the development of a noninvasive and practicable method for quantifying the intrinsic stability of the chemoreflex control of breathing during sleep among the multiple SRBD phenotypes. In this protocol, continuous PAP is applied at a level sufficient to maintain upper-airway patency and abolish inspiratory flow limitation during non-REM sleep. During periods of stable sleep, inspiratory pressure is increased briefly to perturb the ventilatory control system with hyperventilatory “sighs.” The ventilatory responses to these induced sighs that occur without arousal from sleep are subsequently analyzed using a system identification algorithm.
An outcome of this analysis is the estimation of the dynamic loop gain, reflecting the propensity for feedback instability. Preliminary results indicate that primary snorers have significantly higher loop gain than subjects with OSA, an outcome that is opposite to what a recent study has demonstrated in adults, suggesting developmental differences in how loop gain interacts with control of breathing in OSA. The teens then participate in a second night study, in which the upper airways are scanned using DMRI. DMRI provides insight into the development of upper-airway collapse during sleep onset. An intriguing result that has appeared recently is the observation that upper-airway narrowing or collapse occurs even during central apnea, demonstrating that with obesity, constant maintenance of upper-airway muscle tone is needed to keep the airway open. A more detailed account of the application of DMRI can be found in the article, “Seeing Sleep,” by Krishna Nayak and Robert J. Fleck on page 40 of this issue of IEEE Pulse.
Modeling Airways
The group of investigators from the Albert Einstein College of Medicine, University of Pennsylvania, and The Cooper Union for the Advancement of Science and Art are developing novel MRI methodologies and computational models to evaluate the anatomical and functional attributes of the upper airway in children with OSA. The lack of a readily available system to quantify and analyze the upper airway from routine MRI protocols motivated their team to develop a methodology using novel, efficient, and reliable methods. They have developed a new set of algorithms for three-dimensional (3-D) airway construction, visualization, and analysis [1]. This system provides an accurate representation of the airway as it relates to airflow and surrounding tissues and is significantly more efficient than manual segmentation. In addition, they have developed techniques to image the dynamic upper airway using active respiratory-gated, and retrospective, free-breathing MRI that can determine the temporal changes in airway size and shape during the inspiratory and expiratory phases of respiration [2].
A recent development by their group relates to the use of CFD to model the upper airway in children with OSA [3]. The implementation of the above-mentioned methodologies provides for a better understanding of the biomechanical properties of the upper airway that lead to airway obstruction during sleep in children [4] and particularly in groups that are at extreme risk, such as obese children, children with craniofacial abnormalities, and children with neurological deficits. The methodologies could also provide insight into biomechanical properties of the upper airway, leading to OSA in adults, as well as to a better understanding of why residual OSA persists despite conventional management such as AT and upper-airway surgery.
The Einstein team is refining the imaging and computational tools described earlier to evaluate the biomechanical properties of the upper airway in a group of adolescent girls with polycystic ovary syndrome. In this disorder, girls are at a particularly high risk to develop OSA due to endocrine abnormalities and obesity. Currently, they are evaluating how the girls’ upper-airway structure and mechanics, body composition, severity of OSA (determined by sleep studies), and metabolic and inflammatory status (glucose tolerance, insulin resistance, and inflammatory biomarkers) interact and influence each other. The goal is to develop a noninvasive computational model that simulates the upper-airway mechanical properties during sleep. By establishing such a model, they anticipate two major positive outcomes. The first is the development of a novel method for early identification of patient risk for OSA based on MRI images to assess upper-airway mechanics, not only sleep studies. Second, an assessment that is based on imaging and biomechanical properties will direct a more specific approach to the management of such subjects. Thus, it will reduce the possibility of residual OSA and has the potential to be overall more cost efficient.
Quantifying Physiological Dynamics
As we have seen, infants and young children with abnormalities of the upper airway, specifically microagnathia or subglottic stenosis, are at risk for hypoxia, respiratory insufficiency, poor growth, and long-term morbidity. The current state of the art for care includes a multidisciplinary approach, but therapy is typically directed by the clinician’s experience and preference, rather than on normalized physiologic or anatomic metrics. Given this, the decision to pursue surgical intervention versus allowing for improvement related to growth and maturation is often difficult.
As computer technology has become more advanced, biomedical engineering tools are now available for quantifying physiological dynamics in the respiratory tract. The team of researchers coordinated at the University of Indiana and the University of North Carolina is studying 3-D computational models generated from computed tomography (CT) scans. Capitalizing on the fact that these scans are obtained for clinical purposes in patients, they have tapped into a rich database. CFD and fluid–structure interaction models can be generated to compute airflow, wall shear stress, pressure distribution, and the change in airway wall shape during airflow. Furthermore, the computed geometry can be virtually modified to simulate surgical intervention and/or normal growth and development.
To improve the understanding of airway geometry and dynamics during the respiratory cycle, the researchers are developing an optical coherence tomography (OCT) system that can be integrated during flexible and rigid bronchoscopy in young children with upper-airway obstruction [5]. Their long-term goals are to use a functional computational model as a novel, noninvasive clinical tool to evaluate in a quantitative manner abnormalities of the pediatric upper airway; to model and predict the effect of therapies on airway patency and secondary functions, such as speech and swallowing; and to guide medical and surgical management of upper-airway obstructive lesions in children.
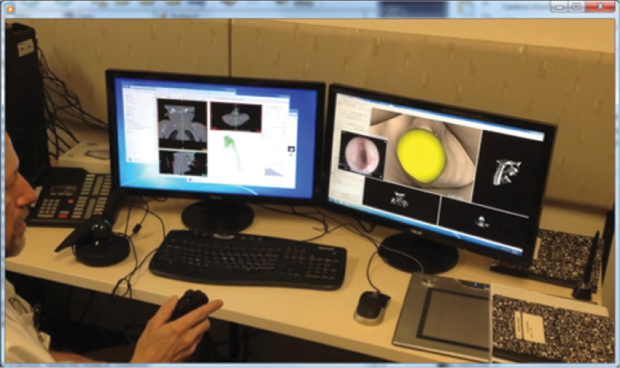
To achieve these goals, they have developed a pediatric airway anatomical atlas to capture the maturation and expected normal variation of the airway based on CT images from normal control subjects [6]. This atlas can be used to assess severity of airway malformations across a wide variety of pathologies and patient populations. They have successfully demonstrated the ability of the atlas to quantify airway narrowing for subglottic stenosis cases. Furthermore, they have developed an integrated virtual pediatric airway workbench (VPAW) to visualize geometries and flows. VPAW also supports editing of the airway and, therefore, could be used for surgical modeling (Figure 3). This tool could be refined to the point that it would simulate the effect of an intended surgery on airway geometry and flow measures, thereby evaluating how surgical modeling ultimately impacts the child’s ability to breathe. In addition, one could also evaluate how growth and watchful waiting impacts the child. Working together with stereo 3-D graphics and force-feedback touch technologies, these techniques would enable a physician to explore multiple potential surgeries to best evaluate the optimal intervention. These options could be shared with patients and families in the virtual format, bringing new weight and meaning to the informed consent process.
Finding the Right Location
The University of California at Irvine (UCI) team aims to develop in vivo measurement and modeling tools for evaluating/predicting upper-airway dysfunction in children. Recognizing that surgery for OSA is extremely challenging, and the success rates are relatively low compared to other operations performed in the upper airway, they have focused on one of the major challenges for surgeons: precisely determining the locations where the airway is prone to collapse. Presently, to accomplish this task, surgeons rely upon endoscopic examinations and generally perform them either during wakefulness in the office or during sedation in the operating room. Diagnostic imaging studies are valuable, but there are limitations with both endoscopy and imaging because of the need for general anesthesia, risk of ionizing radiation, intrinsic limitations in terms of spatial and, temporal resolution, and, of course, cost. All of these factors are substantially amplified in pediatric cases.
The UCI team integrates technical expertise from several fields with expertise in OCT, device design, CFD, and clinical otolaryngology to develop a system capable of generating real-time 3-D volumetric images of the internal airway structure and estimate airflow dynamics in children. The structural information on internal airway anatomy will allow simulation of upper-airway airflow and an estimation of the impact of surgery in relieving airway obstruction. In turn, the model will provide a means to determine which children will benefit from upper-airway surgery, and it is a first step toward developing individualized therapy. The development and rapid translation of patient-specific information from in vivo measurement and modeling will set the stage for presurgical planning/interventional surgery.
Recently, long-range OCT was developed as an anatomic imaging technique that repurposes an OCT system as a rangefinder and can be used to determine the structure of hollow organs within the human body. This team of researchers has developed a high-speed Fourier-domain long-range OCT (FD-LR-OCT) system to achieve real-time 3-D imaging of upper airway from inside the lumen. The system is based on a 50-kHz swept source OCT system that implements an acoustic phase shift to double the imaging range [7]. An imaging probe with a 1.2-mm diameter was designed and developed to acquire structural and anatomical datasets of the human airway. They have successfully translated the FD-LR-OCT system to clinical studies, and have performed in vivo imaging of the upper airway in more than 100 human patients with the high-speed LR-FD-OCT system (Figure 4). High-resolution imaging of the human upper airway from the larynx to the nasal cavity can be completed in fewer than 20 seconds.
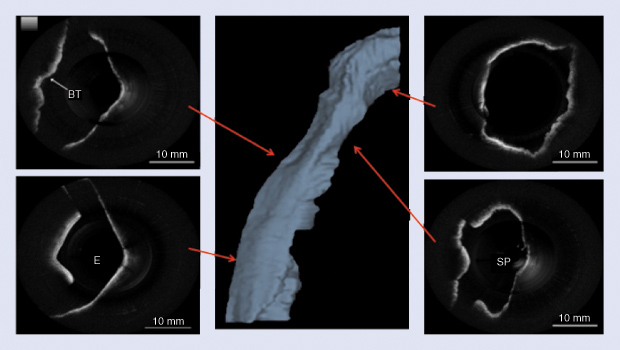
OCT imaging can be gated to the respiratory cycle, which enables imaging of cross-sectional airway geometry with high temporal resolution over the respiratory cycle. Because OCT uses near-infrared light, continuous imaging to quantify airway structure during both asleep and awake states becomes feasible. They are currently building a 400-kHz FD-LR-OCT system that can further increase the imaging speed, which will allow measurements of dynamic changes in the upper airway, critical to the complete delineation of the functional aspects of airway anatomy. Finally, integration of OCT with optical coherence elastography [8] will outline the biomechanical properties of upper-airway tissue, which is essential to understand and manage this complex disorder.
References
- R. Arens, J. M. McDonough, A. M. Corbin, N. K. Rubin, M. E. Carroll, A. I. Pack, J. Liu, and J. K. Udupa, “Upper airway size analysis by magnetic resonance imaging of children with OSA syndrome,” Am. J. Respir. Crit. Care Med., vol. 167, no. 1, pp. 65–70, 2003.
- M. E. Wagshul, S. Sin, M. L. Lipton, K. Shifteh, and R. Arens, “Novel retrospective, respiratory-gating method enables 3D, high resolution, dynamic imaging of the upper airway during tidal breathing,” Magn. Reson. Med., vol. 70, no. 6, pp. 1580–1590, 2013.
- S. C. Persak, S. Sin, J. M. McDonough, R. Arens, and D. M. Wootton, “Noninvasive estimation of pharyngeal airway resistance and compliance in children based on volume-gated dynamic MRI and computational fluid dynamics,” J. Appl. Physiol., vol. 111, no. 6, pp. 1819–1827, 2011.
- D. M. Wootton, H. Luo, S. C. Persak, S. Sin, J. M. McDonough,
C. R. Isasi, and R. Arens, “Computational fluid dynamics endpoints to characterize obstructive sleep apnea syndrome in children,” J. Appl. Physiol., vol. 116, pp. 104–112, 2014. - K. Wijesundara, N. Iftimia, and A. L. Oldenburg, “Design of a swept-source, anatomical OCT system for pediatric bronchoscopy,” in Proc. Soc. Photo Opt. Instrum. Eng., Mar. 2013, vol. 20, p. 8571.
- Y. Hong, B. Davis, J. S. Marron, R. Kwitt, N. Singh, J. S. Kimbell, E. Pitkin, R. Superfine, S. D. Davis, C. J. Zdanski, and M. Niethammer, “Statistical atlas construction via weighted functional boxplots,” Med. Image Anal., vol. 18, no. 4, pp. 684–698, 2014.
- J. Jing, J. Zhang, A. C. Loy, B. J. F. Wong, and Z. Chen, “High-speed airway imaging usingfull-range optical coherence tomography,” J. Biomed. Opt., vol. 17, no. 11, p. 110507, 2012.
- W. Qi, R. Chen, L. Chou, G. Liu, J. Zhang, Q. Zhou, and Z. Chen, “Phase-resolved acoustic radiation forceoptical coherence elastography,” J. Biomed. Opt., vol. 17, no. 11, p. 110505, 2012.



