Organ transplantation has become an established and life-saving treatment for patients with end-stage organ failure. However, patients still face constraints when it comes to access to transplantation, as well as its efficacy. One major concern is the global shortage of organs for transplantation. The World Health Organization estimates that only 10% of the worldwide need for organ transplantation is being met [1] and, according to United Network for Organ Sharing, an average of 20 Americans die each day while waiting for a transplant [2]. In addition, even after an organ is transplanted, long-term outcomes remain uncertain. Half of organ transplants fail within ten years of being transplanted, including as many as 75% of intestines and lungs [1]. To prevent rejection of a donor organ, transplant recipients must adhere to lifelong immunosuppressant drug regimens, the side effects of which include increased risk for life-threatening infections.
To address these limitations, a variety of emerging technologies are focused on either maximizing the availability of organs or minimizing the immune response to donor organs. These arms of research are complementary and may, in the future, be used together to save and improve the lives of organ transplant patients.
“Warm” organ storage
For decades, the gold standard for organ preservation has been to put organs on ice. This method, called static cold storage, keeps organs viable outside the body for several hours by slowing down metabolic processes. But static cold storage only slows, and does not prevent, ischemic injury, which increases the risk of organ dysfunction and other post-transplant complications. The rapidly advancing technology of ex vivo normothermic machine perfusion (NMP), in which organs are kept alive outside the body in a functioning physiological state, is poised to improve the quantity and quality of organs that are suitable for transplantation.
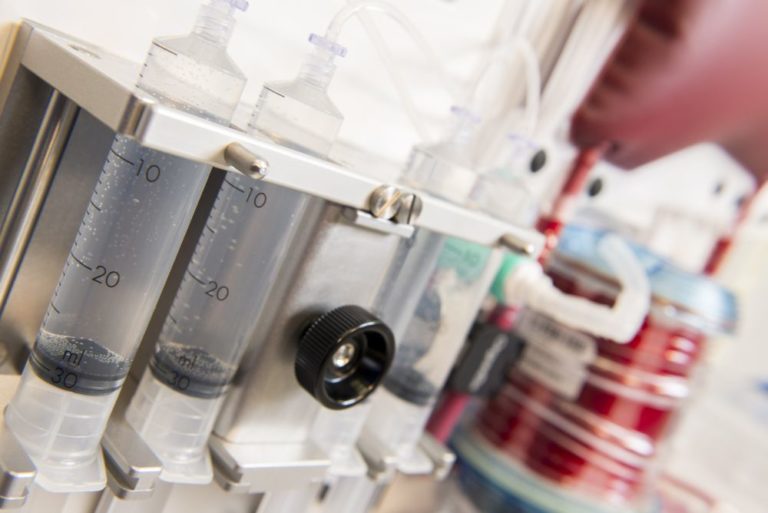
The medical device company OrganOx, founded by University of Oxford researchers in 2008, makes one such NMP device for preserving livers, called the metra [3]. The metra device works by perfusing the liver with oxygenated blood, anticlotting drugs, and nutrients at normal body temperature (Figure 1). The idea is to approximate physiologic conditions and enable the organ to function much as it would inside the body.
In 2018, researchers put the metra to the test against cold storage [4]. The trial involved more than 200 patients across Western Europe who received a liver transplant. Each participant was randomly assigned either a donor liver that had been stored on ice or one preserved with the aid of a metra. The researchers found that livers preserved with NMP showed less damage than those stored on ice.

David Nasralla, a liver transplant surgeon at the Royal Free Hospital in London who co-led the trial, says an even bigger benefit to NMP is that it makes it possible for organs to be functionally evaluated. “Traditionally, surgeons assess organs before transplant simply by looking at them and feeling them,” he says. “It’s incredibly subjective. You show ten different surgeons a liver and ask them whether it’s any good and you’ll get ten different answers.”
This results in a lot of donor organs going unused due to an abundance of caution. But the metra device provides objective data on parameters including blood flow, bile production, and lactate clearance, which tell surgeons more about how the liver might work inside a patient. Nasralla says the device, and others like it, could increase the supply of organs available for transplant by reducing the number that are discarded.
NMP could also provide surgical teams a longer period of time to treat an organ pretransplant, by trimming off fat or administering antibiotics or other drugs. Although a safe time window has not yet been delineated, clinical evidence suggests that organs undergoing NMP are substantially protected from ischemic injury. According to Nasralla, NMP is becoming part of mainstream clinical practice. The metra device is already approved in Europe and OrganOx leases out its devices around the world. In the United States, NMP devices from a few companies are in current and/or recently completed clinical trials, including machines for heart, lung, and liver.
Supercool solution
Meanwhile, a group of Harvard Medical School researchers is investigating an organ preservation method on the other end of the temperature spectrum: supercooling. With static cold storage, donor organs stay viable for several hours at about 4 °C. They could potentially last longer at lower temperatures, but subzero storage leads to the formation of tissue-damaging ice crystals that render organs unusable.
Biomedical engineer Shannon Tessier, an investigator at the Center for Engineering in Medicine at Massachusetts General Hospital, is part of the team that developed the method of supercooling (Figure 2). She was inspired by animals like wood frogs, which freeze solid in the winter, yet thaw and reanimate in the spring with no tissue damage. “Animals that survive extreme temperature fluctuations have evolved strategies that could be applied toorgan preservation,” says Tessier. “We augmented lessons from nature with state-of-the-art technology and have shown that supercooling has promise, at least in a laboratory setting.”

Tessier and her colleagues first pioneered the technology of supercooling in rats in 2014. They demonstrated that it was possible to chill rats’ livers down to −6 °C without any injury to the tissues, extending their preservation time from hours to more than a day. Then, last year, the team reported that they had successfully supercooled human livers down to −4 °C using a cocktail of cryoprotectant chemicals and more elaborate machine perfusion equipment [5].
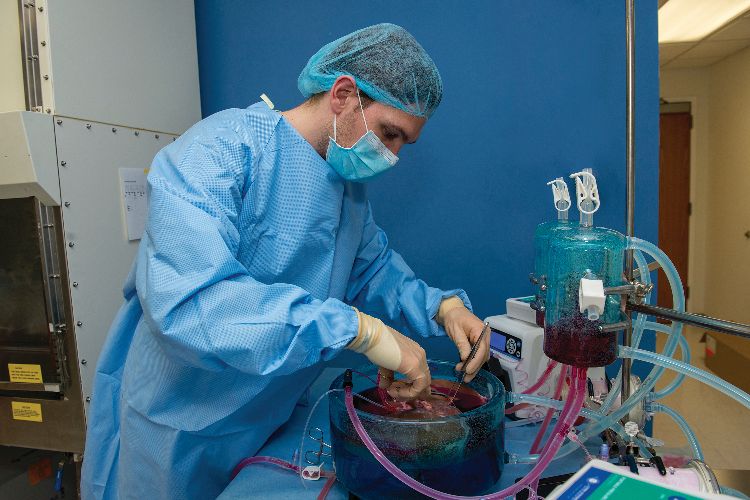
The method involves preconditioning the liver with a preservative solution containing a modified version of a cryoprotectant agent found in wood frogs, as well as additional cryoprotective chemicals aimed at addressing the different modes of injury that can occur as a result of cold storage. This is done via machine perfusion, which ensures that the solution is evenly distributed throughout the organ (Figure 3). The liver is taken off the perfusion machine for storage below zero. Then, at the transplantation site, machine perfusion is again used to remove the preservative agents and warm the livers back to life (Figure 4).
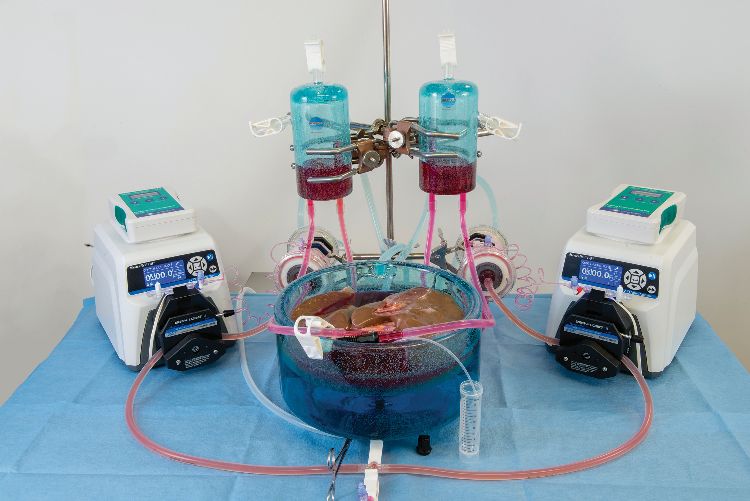
Tessier and her colleagues showed that supercooling could triple a liver’s typical shelf life from 9 to 27 hours. There was no ice formation and, when brought back to body temperature, the livers functioned as expected, based on tests of oxygen use, bileproduction, and lactate metabolism. They also survived a simulated transplant in which they were hooked up to an artificial blood supply. The next step will be transplanting organs preserved via supercooling into large animals such as pigs and assessing how they function.
Tessier thinks both NMP and supercooling will be necessary in the future of organ transplantation. “It is important to note that these organ preservation strategies—both of which depend on machine perfusion—are actually complementary,” she says. “Each one has a different role in the framework of transplantation and each one could help mitigate the organ shortage situation. There might be a time and a place for many different approaches to organ preservation in the future.”
Re-educating the immune system Organ recipients must take a regimen of immunosuppressive drugs for the rest of their lives to prevent their body from rejecting the transplant. Not only is this economically burdensome, the drugs have serious side effects, including increased risk for infections, kidney failure, and cancer.
Researchers around the world are working on therapies to achieve donor-specific tolerance. These therapies include medications and techniques designed to re-educate the immune system to accept the presence of a transplanted organ, while leaving it intact to defend the patient against infections and cancer. Ideally, such therapies would be short-term and eliminate or minimize the need for immunosuppressive medications.
“We know this is conceptually possible,” says Christian Larsen, founding director of the Emory Transplant Center (Figure 5). Larsen cites evidence from experiments in rodents and nonhuman primates, as well as the rare instances in humans where a bone marrow donation has reprogrammed the immune system of a recipient to accept another organ from the bone marrow donor.

Different strategies are under investigation to optimize immune tolerance after transplantation. Many of these involve a kind of white blood cell called regulatory T cells (Tregs). Tregs play a major role in the development of tolerance by suppressing immune responses and preclinical studies have shown that they can delay or prevent transplant rejection [6]. Though promising, there are still many unanswered questions about Treg-based therapies. In the last ten years, a few clinical trials investigating the safety and feasibility of Treg-based therapy have been conducted and published, while many more are ongoing [7]. Results from these ongoing clinical trials will provide guidance as to the role of Tregs in organ transplantation, answering questions about doses, timing, and combinations with other drugs [8].
In the future, immune tolerance treatments may work in tandem with organ preservation technologies. Larsen says advances in organ preservation will create opportunities for recipients to undergo immune tolerance protocols before transplantation, as well as provide time for the organs themselves to be treated. “There are a host of ways one could modify an organ to make it less immunogenic,” he says. “That would potentially be a powerful facilitator for tolerance induction regimens.”
Many challenges to optimize transplant tolerance remain, though Larsen is hopeful. “Our aim is to get one transplant to last a lifetime,” he says. “When I see patients now, my goal is not for this kidney to last for 10 or 15 years. We want it to be more like 40 or 50 years.”
“But then think about taking multiple drugs for the next 40 years. Immune tolerance could potentially achieve a lifelong transplant without the need for all those meds.”
References
- S. Giwa et al., “The promise of organ and tissue preservation to transform medicine,” Nature Biotechnol., vol. 35, no. 6, pp. 530–542, Jun. 7, 2017. doi: 10.1038/nbt.3889.
- F. Messner, Y. Guo, J. W. Etra, and G. Brandacher, “Emerging technologies in organ preservation, tissue engineering and regenerative medicine: A blessing or curse for transplantation?,” Transpl. Int., vol. 32, no. 7, pp. 673–685, Jul. 2019. doi: 10.1111/tri.13432.
- OrganOx. [Online]. Available: https://www.organox.com/
- D. Nasralla et al., “A randomized trial of normothermic preservation in liver transplantation,” Nature, vol. 557, no. 7703, pp. 50–56, May 2018. doi: 10.1038/s41586-018-0047-9.
- R. J. de Vries et al., “Supercooling extends preservation time of human livers,” Nature Biotechnol., vol. 37, no. 10, pp. 1131–1136, Oct. 2019. doi: 10.1038/s41587-019-0223-y.
- M. Romano, G. Fanellie, C. J. Albany, G. Giganti, and G. Lombardi, “Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity,” Front. Immunol., vol. 10, p. 43, Jan. 31, 2019. doi: 10.3389/fimmu.2019.00043.
- Immune Tolerance Network. [Online]. Available: https://www.immunetolerance.org/
- Q. Tang and F. Vincenti, “Transplant trials with Tregs: Perils and promises,” J. Clin. Invest., vol. 127, no. 7, pp. 2505–2512, Jun. 30, 2017. [Online]. Available: https://doi.org/10.1172/JCI90598