The drug development pipeline, once one of the most successful and lucrative commercial sectors in the United States, is now strained by a combination of factors: increased development costs, lengthy time lines, and the poor predictive power of preclinical studies, among others. These factors, in combination with the need to respond to newly evolving demands—including the trend toward personalized or precision medicine, rising rates for many chronic diseases, and continued threats from emerging infectious diseases—are placing extraordinary pressure on an already strained development process.
In response to this situation, there is a growing recognition that new science and new tools will be needed to reduce the costs of drug development and to improve the predictive power of preclinical studies in assessing the safety and efficacy of compounds in the pipeline. Currently, preclinical studies rely most heavily on a combination of experiments in animals (in vivo studies) and measurements using cultured animal or human cells in various formats (in vitro studies), all of which are aimed at assessing the fate of the drug (absorption, metabolism, and interactions) and the drug’s potential toxicity.
The in vivo process is very costly, suffers from inaccuracies due to differences in how drugs affect humans versus various animal models, and raises ethical concerns regarding heavy reliance on animals for this testing. A further drawback of animal models is that mechanistic studies involving precisely controlled experiments and measurements are extremely difficult in living organisms. The in vitro process typically involves drug testing using transformed cell lines arranged in two-dimensional configurations in multiwell plates. This approach suffers from the relatively poor correlation between human responses and those obtained in the artificial environment of a cell culture system, in which neither the cells nor the microenvironments are particularly representative of human physiology.
These deficiencies exhibited by current preclinical models have spurred the development of the field known as organs-on-chips, in which human primary cells are cultured in microfluidic devices designed to mimic key features of the microenvironment of human organs [1]. This article addresses the application of microsystems technology—up to now used primarily for producing microelectronic and microelectromechanical systems (MEMS) devices such as the memory chips and sensors found in mobile phones—to fabricate microscale models of human organs, pump fluids around those organ models much as the heart pumps blood through the body, and sense and measure critical parameters so as to ensure that these organ models operate properly for periods of days or weeks during experiments testing the efficacy and safety of drug compounds.
Microsystems for the Design of Organ Models
One critical deficiency of existing cell culture models used to predict drug behavior in humans is the use of transformed cell lines or cells that are derived from a single source, rather than human primary cells taken from living human organs or tissues. This aspect of cell culture model optimization and development is being addressed through the emergence of systems that utilize human primary cells from a variety of commercial sources, as well as stem-cell-based approaches that can be engineered to represent a broader range of genetic heterogeneity in human populations [2].
These advances on the biological side of organ-model development are being made in parallel with significant progress in the development of microscale organ models that are designed to simulate critical aspects of human tissues and organs. It is here that microfabrication technology plays a key role in the ability to recapitulate the chemical and mechanical microenvironment within human organs, but at a size scale hundreds or thousands of times smaller than the organs these devices are designed to mimic. The reasons for this size reduction are numerous and generally relate to practical limitations in the number of cells, the volume of drug and other reagents required for testing, and the need for multiple high-throughput experiments that drive the economics of the preclinical drug evaluation process.
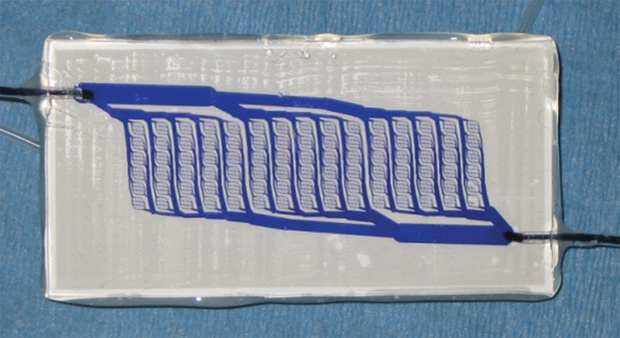
Microfabrication technologies take many forms, but a common approach uses the same photolithographic patterning techniques that form the basis of microelectronic and MEMS device fabrication to create a template for microstructures that can then be replicated in polymer devices. Lithographic patterning is employed to generate a relief pattern on silicon wafers, and these patterned wafers are then used as masters in a process known as replica molding, where a soft and elastic polymer such as silicone or poly(dimethylsiloxane) (PDMS) is cast and peeled from the master and then used to form a transparent polymer structure for cell culture. Multiple layers of these PDMS structures can be cast from various patterned masters and then bonded together and connected to plastic tubing, at which point cells are introduced into various chambers in the structures and fluid. For example, cell culture media can be introduced into the devices to create an artificial tissue or organ construct.
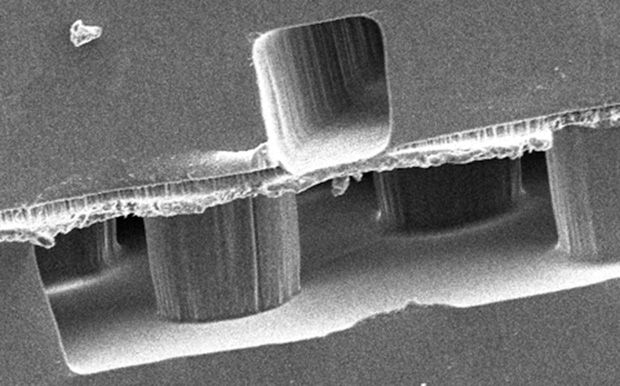
As shown in Figure 1, where a pattern represents a branching network of blood vessels, these patterns can be designed at the microscale or nanoscale to replicate structures in human organs, such as capillary networks. Another example of such a device is shown in the cross-sectional scanning electron micrograph in Figure 2. Here, a membrane bilayer device designed and built using PDMS replica molding from silicon photolithographic masters is assembled to form a dual-compartment system with an intervening semipermeable membrane dividing the two chambers. This fundamental construct has been applied to numerous barrier tissue and organ models, including the kidney, lung, liver, vascular system, and blood–brain barrier. In another example, a lung-on-a-chip comprising an engineered blood vessel network and “breathing” chambers that contain lung-specific human cells is operated by applying alternating vacuum and room-pressure levels to recapitulate the respiration cycle. Studies have shown that effects such as the toxicity of certain types of nanoparticles can be demonstrated using this breathing lung-on-a-chip [3], whereas conventional cell culture models that lack this system’s structural and dynamic aspects do not exhibit such toxicities and so do not correlate as well to human responses.
Microdevices for Controlling Flow
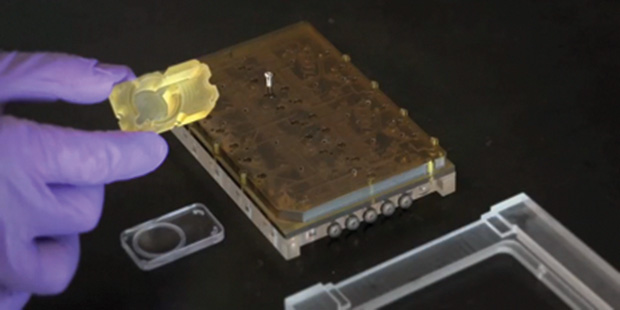
A common feature of virtually all organs-on-chips technologies—and one that distinguishes them from conventional cell culture model systems—is the presence of controlled levels of media flow within and between the organ models. This requirement for flow stems from the rate of oxygen consumption of highly metabolic tissues (such as liver and heart), the importance of fluid mechanical shear stress in governing cell behavior in tissue structures that are exposed to flow (such as vascular and kidney), and the need to replenish and refresh culture media in organ models so as to maintain a healthy physiological state where nutrients and waste products do not reach unacceptably low or high levels, respectively. In addition, the emerging trend toward multiorgan systems in which models of different organs are arranged in an interacting circuit on a platform necessitates fluid exchange to mimic the organ crosstalk that occurs in the body, presenting a significant engineering challenge for fluid circuits that must operate precisely for periods of days or weeks while maintaining fluid levels in each individual well. Figure 3 shows a multiorgan platform in which various organ and tissue models may be placed in a modular or reconfigurable fashion.
Several approaches have been pursued toward the goal of establishing precision fluid flow on organ model platforms for the purposes of perfusing oxygen and nutrients; controlling drug, nutrient, and metabolite concentrations and gradients; and applying fluid shear to cultured cell populations [4]. Early efforts focused on gravity-based effects, largely by rocking the well plate in a manner to realize flow across an organ model and to move fluid from one chamber to another. While this is a convenient and relatively simple method, it lacks precision and cannot provide a wide dynamic range of flow conditions across a highly multiplexed plate of organ models.
Rocking-plate systems have been supplanted to a degree by technologies that leverage capillary-driven flow, where surface tension in small channels is used to provide controlled flow within an organ model and to enable interactions between neighboring wells. Again, while the technique is attractive due to its simplicity, there are limitations in the ability of capillarydriven systems to provide a wide range of flow rates, shear forces, and exchange capabilities due to the strong dependence of the flow on the local geometry.
The limitations of gravity-driven systems have propelled efforts in developing active pumping systems, typically based on pneumatically actuated valves in which a membrane in a pump chamber is deflected due to actuation from an air-pressure line. These pneumatic systems have enjoyed wide use and form the basis of many commercial microfluidic systems—not just in organ model research but in much broader applications for lab-on-a-chip applications. However, while they provide wide dynamic range and precision flow, they involve separate air lines for control of each pump, and this becomes an unwieldy requirement when scaling up to larger numbers of wells in multiplex systems.
In addition, pneumatic systems often contain elastomeric membranes that can become damaged over time or adsorb drugs and other compounds in unpredictable and uncontrolled ways. Another active pumping approach uses robotic dispensing systems that can be easily multiplexed in a manner similar to current instrumentation in many pharmaceutical research labs. These systems essentially enable fluid transfer between wells by withdrawing fluid into a chamber and then dispensing the bolus into a neighboring chamber. While the system is highly scalable, it involves complex instrumentation and does not provide for a full range of continuous or semicontinuous flow rates.
In response to the limitations of these systems, efforts have been aimed at developing a micropumping technology that provides for wide dynamic range and high-precision control over flow rates, eliminates the need in actuation for bulky and unreliable air lines that become unwieldy as systems scale, provides high reliability in a harsh environment, and can be embedded directly into the well plate rather than requiring extensive external instrumentation that raises cost and system complexity. Such a micropump technology has been developed for implantable drug delivery systems [5], which, by definition, require extreme miniaturization and power efficiency while maintaining high reliability during extended operation under harsh temperature and humidity conditions.
The micropump comprises a miniaturized electromagnetic actuator designed to operate in a normally closed condition to minimize power consumption when the pump is not being actuated. The actuator addresses a membrane atop a displacement chamber, and valves situated at the entrance and exit positions enable precise flow control across a range of stroke volumes that can be tuned by changes to the displacement chamber and membrane geometries. The electromagnetic actuator-driven micropumps can be situated across a multiwell plate at high densities; current systems comprise as many as 62 actuators on a single plate roughly the size of a 96-well plate, with only a ribbon cable to provide power and control signals to tether the plate to a computer. Elements of individual microactuators are shown in Figure 4, placed against the backdrop of an actuator plate that houses the actuators in various valving and pumping configurations.

Reliability is achieved through careful design and processing of the stacked microfluidic layers and the materials choices in this micropump architecture, as well as by housing the critical components in a hermetically sealed manner. Ultimately, these systems can be rendered completely untethered by integrating the power and electronic control into the plate itself, making these instrumented well plates essentially dynamic, “smart” versions of current conventional plastic well plates for cell culture applications.
Microsensors for Measurement and Control
Microsensors based on MEMS technology are having an enormous impact in a wide range of industries and applications, including inertial sensors in automotives, mobile phone devices, and gaming systems; pressure sensors in several biomedical applications; and chemical sensors for industrial and security purposes. One of the most powerful opportunities for microsystems technology in the drug development process is the ability of microsensors to monitor and control organ models in a real-time manner.
Currently, automation technologies capable of monitoring cell culture conditions in multiwell plates focus on assessment of morphology, cell growth, and protein expression, using a variety of markers and stains that provide basic feedback on cell viability and other parameters. However, with the advent of organ model systems in which environmental conditions and cues such as flow, gradients of oxygen and other nutrients, and matrix composition and mechanics can be varied and precisely controlled, techniques for monitoring these parameters in real time are urgently needed. In response to these emerging needs, several types of microsensors for real-time monitoring of conditions in a higher throughput or multiplex manner are being developed.
One class of microsensor technology that is finding wide use in organ models and systems provides measurement of basic cell culture parameters such as oxygen, temperature, pH, and molecular measurements, including glucose and lactate. A key aspect of these sensors is miniaturization and simplification of the sensor architecture and the routing of signals in multiwell plates to improve the reliability and robustness of the system and to reduce the impact of the sensor network architecture on other properties of the system, including the ability to retain high-resolution optical access. Many of these sensor architectures leverage patterning techniques from MEMS fabrication processes, such as the formation of interdigitated electrode geometries and various chemistries using photolithographic techniques and sputter- and chemical-vapor-deposited films.
As described previously, one of the most critical aspects of organ model systems is the ability to provide controlled levels of flow for perfusion and to impart fluid shear stress on cultured cell populations. Flow sensor technologies based on the calorimetric principle can be deployed on multiwell plates to monitor pump rates over periods of weeks as needed during extended operation of these organ model systems. Progress in flow sensor technologies for applications in biomedical devices such as insulin delivery systems and ventilators can be leveraged toward multiplex configurations where flow sensors are integrated in line with the micropumps to ensure stable operation and to detect any problems during experiments that otherwise might be misinterpreted as biological issues with the cells or drug responses.
More specialized sensor measurements in organ model systems can be very useful in assessing tissue responses to drugs and other stimuli in various preclinical studies. An example with wide application is the measurement of trans-epithelial electrical resistance (TEER) to monitor the health of barrier tissues in organ models and systems. These measurements are highly useful in assessing the properties of models of the lung, intestine, skin, and other tissues, as well as to monitor changes in TEER over time (during operation of organ model systems) and across responses to inflammatory insults and drug dosing in these experiments. Conventional monitoring of TEER is manual, and requires interruption of operation of the cell culture, removal from the incubator (and the resulting disturbance of the cell culture), and the potential for contamination. Therefore, an automated measurement of TEER in a multiplex organ model system would increase reliability and provide critical information on the status of the model and the response to drug dosing. Recent efforts point out that TEER measurements in microfluidic systems present specific challenges [6] and are highly sensitive to small changes in the confluence of cells cultured on barrier membranes. These measurements thus present a powerful opportunity for real-time monitoring using microfabrication and microsensing technology.
Summary
This article described a range of opportunities for microsystems technology to address and positively impact the challenging environment of the drug development process. Deficiencies in the ability of animal studies and currently existing simplified cell culture systems to accurately predict the safety and efficacy of compounds in the drug development pipeline can be addressed by a combination of advances in cell biology and in the domain of microengineered systems. Toward this latter end, a combination of emerging capabilities in microfluidic design and fabrication techniques, microactuator and pump technologies, and microsensors and integration methods, presents a powerful opportunity to create a new generation of physiologically relevant, precisely controlled, and scalable engineered systems for use in the drug development process.
References
- S. N. Bhatia and D. E. Ingber, “Microfluidic organs-on-chips,” Nat. Biotechnol., vol. 32, no. 8, pp. 760–762, 2014.
- A. Ranga, N. Gjorevski, and M. P. Lutolf, “Drug discovery through stem cell-based organoid models,” Adv. Drug Deliv. Rev., vol. 69– 70, pp. 19–28, 2014.
- D. Huh, D. C. Leslie, B. D. Matthews, J. P. Fraser, S. Jurek, G. A. Hamilton, K. S. Thorneloe, M. A. McAlexander, and D. E. Ingber, “A human disease model of drug toxicity-induced pulmonary edema in a lung-ona- chip microdevice,” Sci. Transl. Med., vol. 4, no. 159, p. 159ra147, 2012.
- N. K. Inamdar and J. T. Borenstein, “Microfluidic cell culture models for tissue engineering,” Curr. Opin. Biotechnol., vol. 22, no. 5, pp. 681–689, 2011.
- V. Tandon, W. Kang, A. J. Spencer, E. S. Kim, E. E. L. Pararas, M. J. McKenna, S. G. Kujawa, M. J. Mescher, J. Fiering, W. F. Sewell, and J. T. Borenstein, “Microfabricated infuse-withdraw micropump component for an integrated inner-ear drug-delivery platform,” Biomed. Microdevices, vol. 17, no. 2, p. 37, 2015,
- M. Odjik, A. D. van der Meer, D. Levner, H. J. Kim, M. W. van der Helm, L. I. Segerink, J.-P. Frimat, G. A. Hamilton, D. E. Ingber, and A. van den Berg, “Measuring direct current trans-epithelial electrical resistance in organ-on-a-chip microsystems,” Lab Chip, vol. 15, no. 3, pp. 745–752, 2015.