Neuromodulation techniques using magnetic fields and electrical stimulus date back to before the 19th century. Presently, neuromodulation is achieved with various modes of physical stimulation including magnetic, electrical, acoustic, optical, and even thermal stimulation to neural systems. These techniques have been used in clinical treatment for mental disorders (e.g., depression) and movement disorders (e.g., Parkinson’s disease) [1]. In this article, we will give an overview of the typical neuromodulation techniques and the novel techniques proposed in recent years.
In 1985, Anthony Barker and his colleagues reported their remarkable neuromodulation study in which they activated the neural activity of a targeted cortex and successfully induced contralateral hand movements by stimulating the motor cortex with a magnetic field generated by a coil (Figure 1a). This technique, called transcranial magnetic stimulation (TMS), modulates neural activity noninvasively by delivering magnetic waves penetrating through the scalp and skull to the targeted brain regions. Repetitive TMS (rTMS) can either excite or inhibit cortical activity by specific parameters respectively, and the induced effects may last for a period of time after stimulation. For example, low frequency (1 Hz) rTMS to the motor cortex may suppress its cortical excitability, resulting in a transient “virtual lesion” on motor function. The “virtual lesion” induced by rTMS has been used to investigate various cognitive processes including attention, working memory and decision making. TMS has also been widely applied in clinical studies for mental disorders, and has been used as a treatment technique for drug-resistant depression and migraines approved by the Food and Drug Administration (FDA).
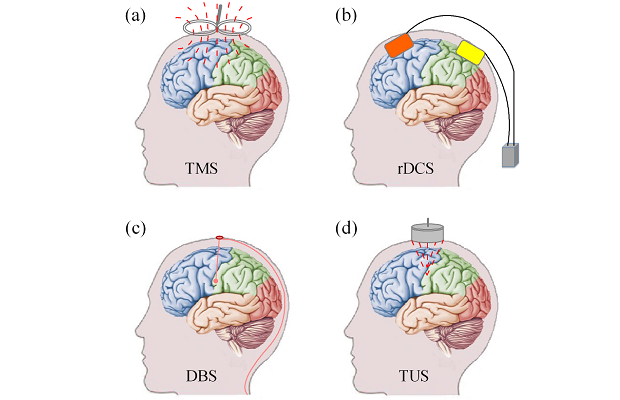
Transcranial direct current stimula¬tion (tDCS) is a relatively simple neuromodulation technique that delivers weak direct current (usually 1-2 mA) with an anodal electrode and a cathodal electrode attached to the scalp (Figure 1b). Stimulation by tDCS could enhance the performance of a variety of cognitive tasks including language learning and working memory. One limitation of tDCS is its relative poor spatial resolution, that is, stimulation by tDCS is not focused enough to a small brain region. Generally, tDCS of a smaller-sized electrode would generate more focused stimulation. Recently, high definition tDCS (HD-tDCS, with multiple small-sized electrodes) has been proposed and may lead to greater and longer lasting effects than traditional tDCS. Compared with TMS, tDCS is more economical, but its neuromodulation effect is weaker, as tDCS only increases spontaneous cell firing while TMS induces the firing of neuron action potentials.
Deep brain stimulation (DBS), introduced in 1987, modulates neural activity by delivering electrical impulses through electrodes implanted to specific brain regions (usually the brain nucleus) for the treatment of movement and mental disorders (Figure 1c). Though DBS is an invasive technique and may cause side effects such as apathy, hallucinations and depression, it offers good therapeutic benefits for several neurological diseases, and has been approved by the FDA as treatment for essential tremor in 1997, Parkinson’s disease in 2002, dystonia in 2003, and obsessive-compulsive disorder in 2009. Moreover, studies also demonstrate that DBS may be a promising treatment technique for chronic pain and other mental disorders like treatment-resistant depression.
Though TMS, tDCS, and DBS have been widely applied in both neuroscience and clinical treatment for mental or movement disorders, they have their own limitations. TMS has relative low focality (~ 2 cm^2) and penetration depth, and thus could only work for the superficial cortex, and cannot reach deeper brain structures like the hippocampus. The traditional tDCS has even lower focality and penetration depth than TMS. DBS have high focality and penetration depth, but requires surgical placement of the electrodes to the target brain regions and is expensive. In 2010, transcranial ultrasound stimulation (TUS) was proposed, and has been demonstrated the potential to address these challenges.
TUS modulates (excites or inhibits) the neural activity by non-invasively delivering specific pulsed ultrasonic waves through the intact scalp and skull to the targeted brain regions (Figure 1d). Ultrasound is a kind of mechanical wave with frequencies above the range of human hearing (>20 kHz). The TUS system is relative simple and would not be more expensive than TMS. A typical TUS system consists of a signal generator that generates electrical signal sequences, a ratio frequency amplifier to amplify the signal sequences from the signal generator, and a piezoelectric transducer to convert the electrical signal into ultrasonic waves. TUS with focused ultrasound has higher focality (~mm) and deeper (>2cm) penetration depth than TMS and tDCS, and is noninvasive compared with DBS. With these advantages, TUS has drawn great attention in both basic neuroscience and clinical research in the past several years. Studies of TUS on animals including rats, rabbits, sheep, and monkeys have demonstrated TUS does not induce obvious harm in brain tissues and behaviors. What’s more, TUS also demonstrated good therapeutic effects for animal models for diseases like Alzheimer’s Disease and epilepsy, and has successfully modulated the activities of the primary somatosensory cortex and enhanced the performance on sensory discrimination tasks of humans [2].
Optogenetics is an innovative neuromodulation technique developed in the past decade, and has been considered one of the most exciting techniques in neuroscience (highlighted in the article on “Breakthroughs of the Decade” in the journal Science in 2010). Optogenetics uses genetically modified individual neurons to express light-sensitive ion channels (e.g., channelrhodopsin (ChR)-2), which is just like a switch that is added to “open” (excite) and “close” (inhibit) the activities of the neurons, where the switch (i.e., light-sensitive ion channels) is controlled by specific light. The activities of the neurons could be altered by light in vivo with high spatial (single neuron) and temporal (~millisecond) resolution.
To modulate the activities of superficial brain regions, light could be delivered by optical fibers or LEDs mounted to the skull of the animal. To gain deeper neuromodulation, light could be delivered through fibers that penetrate into the targeted deep brain regions. Furthermore, by combining the wireless technique with the LED module mounted to the head, optogenetics has been used to study the complex behaviors of freely behaving animals. Studies based on optogenetics have enriched our understanding of fundamental neuroscience problems such as how specific neurons contribute to neural circuits (e.g., mapping the neural circuits in the amygdala that are related to fear) in vivo, and also provided new insights for neurological diseases (e.g., Parkinson’s disease) and mental disorders (e.g., autism, schizophrenia, and depression).
Similar to optogenetics, two new techniques called magnetogenetics and sonogenetics were proposed in 2015. Magnetogenetics could achieve noninvasive neuromodulation by remote magnetic stimulation to neurons genetically expressed with exogenous magnetoreceptor (an iron-sulfur cluster assembly protein 1). Since a magnetic field could penetrate into deep brain regions noninvasively, magnetogenetics seems to have some advantages over optogenetics. A study of sonogenetics found that TRP-4, a pore-forming subunit of a mechanotransduction channel, is sensitive to low-pressure ultrasound stimulation, and C. elegans missing the TRP-4 mechanosensitive ion channel is less sensitive to the ultrasound-microbubble stimulation. What’s more, misexpressing the TRP-4 ion channel in specific neurons would change (increase or suppress) their neural activity and behaviors under ultrasound stimulation. Though the seminal studies on magnetogenetics and sonogenetics only reported the neuromodulation effect in C. elegans, magnetogenetics and sonogenetics could be improved to have great potential in a broad range of basic and translational neuroscience research, considering the advantages of non-invasiveness and deep penetration.
The above neuromodulation effects are all gained directly by physical stimulation. In recent years, various nanomaterial-enabled neural stimulation techniques were proposed based on transduction schemes between electric field, magnetic field, optical waves, and ultrasonic waves, which provide the possibility to overcome the limitations of neuromodulation techniques based on only physical stimulation (Table 1) [3]. For example, to date, the light-sensitive ion channels used in optogenetics are mostly reactive to visible light, which cannot penetrate into deep brain tissue without implanted optical fibers. Fortunately, brain tissues are almost transparent to the near-infrared (NIR) light (650-1450 nm), which is within the so-called ‘imaging window’. Taking this advantage of NIR, a recent study proposed a NIR optogenetics technique based on an opto-opto up-conversion, and enables deep stimulation without planting fibers. This technique uses lanthanide nanoparticles (LNPs) to absorb low-energy NIR light (the primary stimulus) and then act as luminous bodies to emit high-energy visible light (second stimulus) to activate the near-by neurons expressed light-sensitive ion channels. Another recent study proposed a minimally invasive and remote neuromodulation technique based on magneto-thermal transduction. Under the alternating magnetic field (i.e., on/off, 15 kA/m, 500 kHz), the nanoparticles injected into the ventral tegmental area (VTA) generate heat by hysteresis, and thus alter reversible firing of the neurons expressing heat-sensitive TRPV1+ in VTA. The effects of chronic neuromodulation by this technique was validated in mice over a month.
Usually, the primary stimulus should be able to penetrate skull and brain tissues deeply and be safe for exposure, and the specific nanoparticles need to convert the primary stimulus to the secondary stimulus with an adequate amount, so as to activate or inhibit the target light-, voltage-, or temperature-sensitive ion channels in the membrane of neurons (Table 1). Note that nanomaterial-enabled neuromodulation techniques are still in their early states; most of them have only been attained in vitro, several of them have been validated in animals. Before these techniques are applied in the human brain, many more studies are needed to address issues that include biocompatibility, consistency, efficiency, deleterious effects induced by genetic modification to the target neurons, and long term safety.
[accordion title=”Table 1. Typical Nanomaterial-Enabled Neural Stimulation”]
| Transduction | Primary Stimulus | Second Stimulus | Nanomaterial | Working Mechanism |
| Opto-electric | Light | Electric field | Quantum dots | Voltage-gated ion channels |
| Opto-opto | Near-infrared Light | Light | Ianthanide nanoparticles | Optogenetics |
| Opto-thermal | Light | Heat | Gold nanomaterials | Temperature-sensitive inhibitory TREK-1 channels |
| Magneto-electric | Magnetic field | Electrical field | Magneto-electric nanoparticles | Voltage-gated ion channels |
| Magneto-thermal | Magnetic field | Heat | Superparamagnetic nanoparticles | Neurons expressed the temperature-gated TRPV1 ion channels |
| Acousto-electric | Ultrasound | Electric field | Piezoelectric nanomaterials | Voltage-sensitive channels |
[/accordion]
References
- M. D. Johnson, H. H. Lim, T. I. Netoff, A. T. Connolly, N. Johnson, A. Roy, A. Holt, K. O. Lim, J. R. Carey, J. L. Vitek, and B. He, “Neuromodulation for brain disorders: challenges and opportunities,” IEEE Trans Biomed Eng, vol. 60, pp. 610-24, Mar 2013.
- W. Legon, T. F. Sato, A. Opitz, J. Mueller, A. Barbour, A. Williams, and W. J. Tyler, “Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans,” Nature Neuroscience, vol. 17, pp. 322-329, Feb 2014.
- Y. Wang and L. Guo, “Nanomaterial-enabled neural stimulation,” Frontiers in Neuroscience, vol. 10, 2016-March-7 2016.



