Bioprinting is an additive manufacturing process used to create architectures that mimic natural living tissues in form and function [1]. It involves the deposition of bioink, which can include a mixture of living cells, nutrients, and extracellular matrix. The bioink is then deposited onto a scaffold to generate 3-D structures that imitate natural tissues and organs. This process has already been used to generate a diverse range of products, including bioprinted human ears for transplant, and 3-D printed bioceramic and modified biopolymer bone implants that received U.S. Food and Drug Administration (FDA) marketing approval [2]. Researchers are working on bioprinted versions of a wide range of organs, including liver, kidney, lung, and heart.
Bioprinting has the potential to revolutionize organ transplantation by directly addressing issues such as the shortage of transplantable organs. Interest in bioprinting has seen explosive growth in both academia and industry, as evidenced by the increased number of published articles and valuations of the industry [1]. The global bioprinting industry was valued at $586.13 million in 2019 and is expected to triple in size to $1.9 billion by 2025 [1].
Advances in bioprinting are beginning to generate products that are indistinguishable from natural materials. For example, early in 2022, a patient received a 3D bioprinted ear implant formed from her own cells [3]. This type of technology is important because it does not suffer from the transplantation rejection that is present when using a third-party organ. In another example, a 3D printed human lung scaffold capable of gas exchange in animal models was introduced as the “world’s most complex 3-D printed object,” which included 44 trillion parts (voxels) providing 4,000 kilometers of pulmonary capillaries and 200 million alveoli shown in Figure 1 below [4]. One of the goals is to produce transplantable human lungs by introducing a patient’s own stem cells into the 3-D-printed scaffold.
How can bioprinting breakthroughs be protected with patents?
While each country has its own patent laws that may impact the scope of protection available for various different technologies, one requirement under U.S. patent law is the “patent eligibility” requirement, a threshold question that determines whether subject matter is patentable (assuming it is also novel and unobvious and meets other patent requirements). The U.S. Supreme Court has expanded certain categories of ineligible subject matter that may restrict the extent to which bioprinting breakthroughs can be patented. For example, in Association for Molecular Pathology v. Myriad Genetics, Inc., 569 U.S. 576 (2013), the Supreme Court found that human gene sequences are not patent eligible because they are products of nature. As the products of bioprinting become less distinguishable from unmodified products of nature, they will likely encounter patent eligibility issues [5].
There are, however, many aspects of bioprinting that are clearly patent eligible, as well as potential strategies that may allow U.S. patents to be granted even on the end products. First, processes of bioprinting that involve a series of steps, together with controlled conditions that enable the assembly of structures, are patent eligible. Second, the hardware and reagents (printing devices, components of the printers, novel bioinks that contain nonnatural ingredients, etc.) are patent eligible. Finally, even the end products may be patentable subject matter to the extent that they have a distinct structure or contain nonnatural ingredients. For example, a bioprinted organ may include human cells printed over a scaffold containing a nonhuman ingredient like alginate. This type of end-product may be patent eligible in the USA because the combination of alginate and particular human cells in one product does not exist in nature.
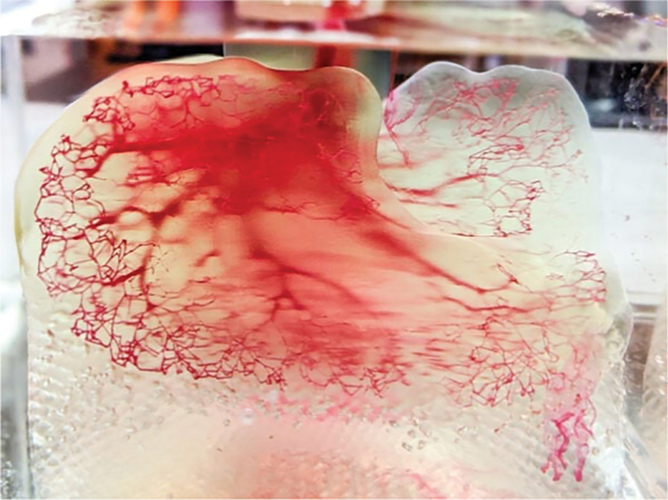
Figure 1. Bioprinted lung scaffold. (Photo courtesy of 3D Systems.)
Another potential strategy that inventors in this field may consider is a “product-by-process” claim. When the process of bioprinting confers unique structural differences in the product, then the end product may be patentable as a product-by-process claim. To take a specific example, if the process leads to differences in the density of cells or differences in the tubular support scaffold architecture compared to the naturally occurring products, then product-by-process claims may be appropriate. In addition, the bioprinted end products may be perfused with nonnaturally occurring fluids to preserve and stabilize them before they are transplanted, and so the perfused preimplanted bioproduct may also give rise to a separate set of patent claims that are patent eligible.
The U.S. Supreme Court’s Myriad decision narrowed the doctrine of patent eligibility for products of nature. However, even the Myriad court recognized that a nonnaturally occurring product is patentable. Accordingly, there are a number of pathways available to obtain patent protection for bioprinting breakthroughs. Additionally, the U.S. Congress is currently considering whether to modify the patent eligibility statute in a way that may roll back the Supreme Court’s decision, perhaps opening up even more avenues for patenting bioprinting breakthroughs.
References
- S. Santoni et al., “3D bioprinting: Current status and trends—A guide to the literature and industrial practice,” Bio-Des. Manuf., vol. 5, pp. 14–42, Dec. 2022.
- A.D.A.M. Receives 510 (K) Eligibility From FDA for 3D Printed Bones. Accessed: Dec. 1, 2022. [Online]. Available: https://3dprintingindustry.com/news/a-d-a-m-receives-510-k-eligibility-from-fda-for-3d-printed-bones-177805/
- Cornellian-Founded Company Implants 3D-Bioprinted Ear. Accessed: Dec. 1, 2022. [Online]. Available: https://news.cornell.edu/stories/2022/06/cornellian-founded-company-implants-3d-bioprinted-ear#:~:text=The%20cells%20are%20expanded%20in,shape%20of%20a%20typical%20ear
- United Therapeutics and 3D Systems Shoot for 3D Printed Lung Scaffold Trials Within Five Years. Accessed: Dec. 1, 2022. [Online]. Available: https://3dprintingindustry.com/news/united-therapeutics-and-3d-systems-shoot-for-3d-printed-lung-scaffold-trials-within-five-years-210303/
- N. M. Althabhawi and Z. A. Zainol, “The patent eligibility of 3D bioprinting: Towards a new version of living inventions’ patentability,” Biomolecules, vol. 12, no. 1, p. 124, Jan. 2022, doi: 10.3390/biom12010124.



