The test will be part of a large U.S. study to understand prevalence, post-infection immunity and potential lingering health impacts.
An at-home test for coronavirus disease 2019 (COVID-19) could be released commercially as early as August, according to Scanwell Health of Los Angeles. A combination of a finger-prick blood sample and a smart phone app, the test is designed to detect the presence of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19. The company hopes to receive Emergency Use Authorization from the U.S. Food and Drug Administration (FDA) by the end of summer, and make its first commercially available tests soon after.
Those same at-home COVID-19 tests are now being deployed as part of a major study beginning in the eastern United States to gain a clearer picture of the prevalence of COVID-19 in the general population, and such unknowns as whether and how long recovered patients are protected from reinfection, or if infection carries any long-lasting health impacts.
Testing at home
The COVID-19 test is one of a series of at-home tests in Scanwell’s portfolio, including one for urinary tract infections that went on the market in 2018 [1], and others in development for chronic kidney disease [2] and malaria, according to Jack Jeng, M.D., chief medical officer at Scanwell (Figure 1). The company turned its attention to a coronavirus test in March, after watching China scramble to diagnose COVID-19 with not only polymerase chain reaction (PCR) tests, but also rapid serological antibody tests. “We looked at the rapid serology tests the Chinese were using in labs and hospitals, and felt it was straightforward enough to bring it into the home with the right instructions and software, which is where we have experience,” he recalled.
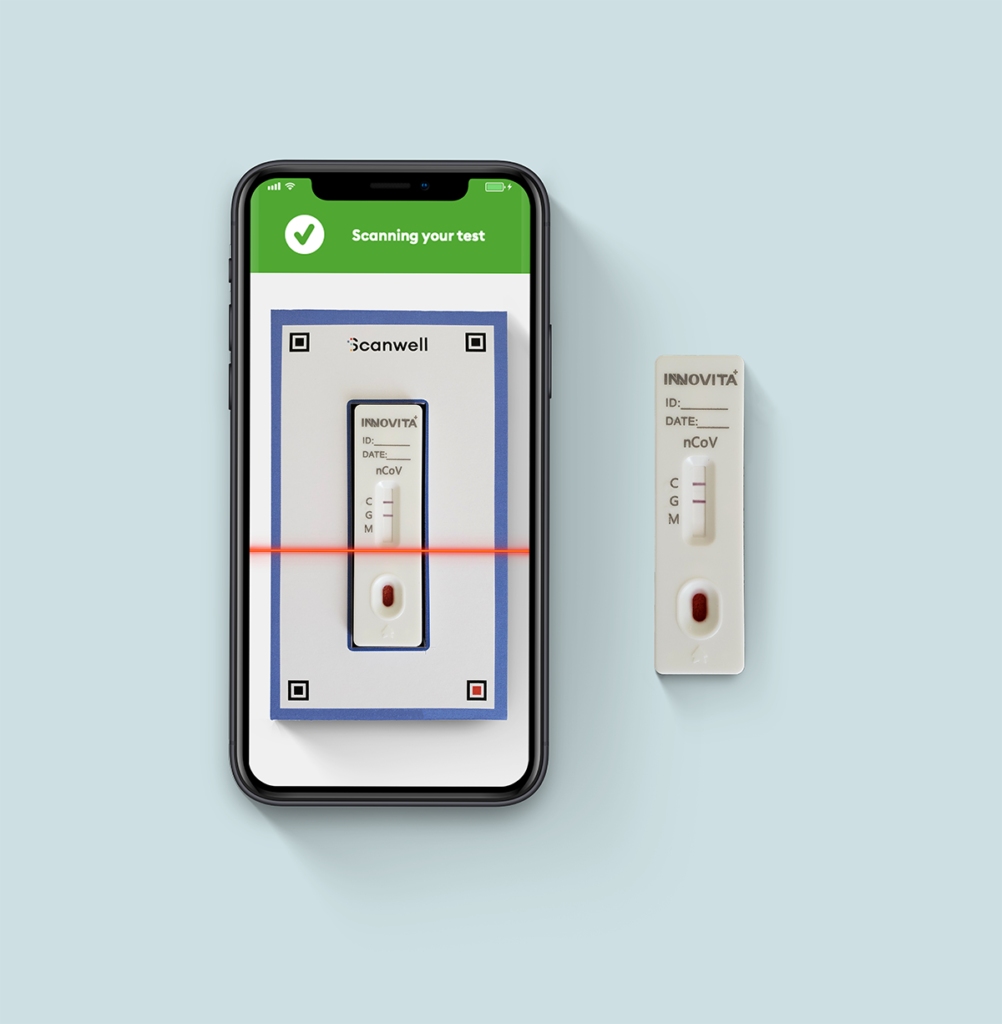
Scanwell got in touch with the Chinese company Innovita that had developed the first China FDAcleare rapid serology COVID-19 test, which has been widely distributed in hard-hit areas, including Wuhan, China. The Innovita test is a sandwich immunoassay that uses lateral-flow technology: small blood sample from the patient is placed onto a test cassette that contains antigen, or proteins that have been grown in the lab to target two antibodies— immunoglobulin M (IgM) and immunoglobulin G (IgG) that the body produces in response to SARS-CoV-2 exposure. If the antibodies are present, they latch onto the antigen, and the test registers positive for virus exposure. Scanwell is also working with other manufacturers to produce similar cassettes and help guarantee against a lapse in supply.
Scanwell then built a full point-of-care test kit that includes a test cassette, lancet, alcohol pad, blood-collection device, diluent, and scan card. In all, it works like this (Figure 2):
- The patient completes an online questionnaire with a doctor or nurse practitioner at the San Francisco, CA-based telehealth provider Lemonaid Health [3]. If appropriate, the provider orders the test to be mailed to the patient.
- The patient downloads Scanwell’s app, which provides step-by-step directions for performing the test.
- The patient pricks a finger with the provided lancet, collects a drop of blood, transfers it onto the test cassette, and adds two drops of diluent.
- After a 13-minute timer, the patient places the cassette on the Scanwell scan card, the app captures an image using computer vision technology to make sure lighting conditions are appropriate, and transmits the image to a health care provider at Lemonaid for analysis, interpretation, and final consultation with the patient.
Jeng anticipates that the company will charge about $30 plus shipping for the at-home commercial test (Lemonaid charges an additional fee for the telehealth consultation). He adds, “This virus is something that affects rich and poor, old and young, anybody and everybody, so we want to make the test as accessible as possible and offering the test at an affordable price is a big part of that.”
Testing broadly
On April 13, Scanwell began sending out its at-home COVID-19 test kits as part of the COVID-19 Community Research Partnership study starting up in North Carolina and parts of neighboring states [4].
The study includes conducting longitudinal antibody testing of 25,000 people over a 12-month period as a way to determine prevalence of the virus in the general population. Study participants will receive both the Scanwell test, and a blood-draw kit made by Neoteryx of Torrance, CA [5], according to John Sanders, M.D., chief of infectious diseases at Wake Forest Baptist and principal investigator of the study (Figure 3). For the blood draw, the patient simply takes a microsample of blood and mails it to the lab, which does all of the analysis. By adding the more traditional lab analysis, the study will help ensure the accuracy of the newer Scanwell test, he said. “This will not only allow us to validate the lateral- flow assay approach that is part of the Scanwell test, but it will also allow us to do deeper analysis by looking at other antibodies (beyond IgM and IgG) and immune markers that may be present, which we think will give us the leeway over the course of the year to ask and answer questions about which antibody predicts protection against disease, which cytokines are necessary to predict good or bad outcomes for disease, and what are we ultimately driving toward with development of a vaccine, biologics or therapeutics.”

The study will also be recruiting 500,000–750,000 patients diagnosed with COVID-19 to gather information about their symptoms, COVID contacts, other health conditions, and medications they may be taking, as well as obtaining access to their electronic medical records, notably various COVID-related lab results. Patient recruitment will begin in the Wake Forest Baptist Health and Atrium Health of Charlotte systems, and potentially expand to health systems elsewhere in the United States, Sanders said.
As the study began, North Carolina was relatively early in the epidemic compared to New York or other parts of the country, Sanders noted, so it was a good place to monitor the arc of the infection in real time, particularly as businesses opened. He also anticipated that it would reveal how long immunity lasts following infection, whether patients who mount weaker antibody responses are less protected from reinfection, and whether infection may lead to health consequences, such as an increased risk of heart attack or stroke.
While he is certain this study will provide vital information, Sanders readily acknowledges that it will fall short of typical study conventions. For instance, he and the other coprincipal investigators expect they may have to make adjustments to the study protocol in the coming months, perhaps switching from doing antibody testing once a month to once every three months due to costs, or changing sampling methodology so they can ensure a good representation among minority populations. “We own those limitations but because we’re in a pandemic, we felt the urgency to start collecting data outweighed the need to have purity of data,” he commented. “We are building the plane as we’re flying the plane, and so we know that as we go forward, we’ll be doing some editing.”
Both the study group and Scanwell have felt the rush to get work done, but were also compelled to do their part in the fight against the novel coronavirus. Jeng remarked, “It’s very easy to feel helpless when you’re trapped at home watching and reading all the news about what’s happening and feeling like you have no control and no way to do anything about it, but I think our whole team really rallied around this idea of doing something that can actually make a difference. All of us are just excited and grateful for the opportunity to help.”
References
- Scanwell Health, “Test and treat UTIs from your smartphone: Know if you have a UTI in 2 minutes. Get same-day prescription for antibiotics,” Accessed: Apr. 21, 2020. [Online]. Available: https://www. scanwellhealth.com/
- Scanwell Health, “Improving screening and monitoring for kidney disease at home: National multi-center study of 1,250 patients with chronic kidney disease,” Accessed: Apr. 21, 2020. [Online]. Available: https:// www.scanwellhealth.com/chronic-kidney-diseasestudy
- Lemonaid Health, Accessed: Apr. 21, 2020. [Online]. Available: https://www.lemonaidhealth.com/
- Wake Forest Baptist Medical Center, “Wake Forest Baptist, Oracle, Javara launch communitybased COVID-19 research study,” press release, Apr. 13, 2020. Accessed: Apr. 21, 2020. [Online]. Available: https://newsroom.wakehealth.edu/News- Releases/2020/04/Wake-Forest-Baptist-Oracle- Javara-launch-COVID-19-research-study
- Neoteryx, Accessed: Apr. 21, 2020. [Online]. Available: https://www.neoteryx.com/