Although doctors still cannot simply order new, functioning organs for patients who need replacements, researchers in labs around the world are making the important advances in tissue engineering that set the stage for regenerative medicine as well as make other biomedical technologies possible.
The breadth of current projects reflects the enormous interest in tissue engineering from both the basic and applied sides, all fed by a swiftly expanding understanding of cellular and tissue structure and function, and always-improving technological capabilities. Some labs focus on the fundamental side, looking into such questions as how to create three-dimensional (3-D) tissues or how to introduce vascularization; others are developing technologies they hope will begin to transform clinical care. In both cases, engineering, biology, chemistry, and multiple other fields are coming together to push the work forward.
Building the Foundation
Most current work is performed in two dimensions and involves trying to build small tissue constructs on a silicon chip or in a Petri dish. These two-dimensional (2-D) projects have served as important steps in the quest to develop organs- on-a-chip or labs-on-a-chip for various purposes, notably to find and test potential new drug therapies.

These small 2-D constructs have been critical to progress in tissue engineering, but they aren’t perfect representations of real, living biological tissues. “These on-a-chip technologies are still being developed, and while they generate a lot of excitement, there’s only so much tissue you can fit on a chip—usually a couple of cell layers,” says Deb Nguyen, Ph.D., senior director of research and development at Organovo of San Diego, California (Figure 1, right). “Because you don’t have real 3-D tissue architecture, you can’t get all the readouts you want or need from them, and you can’t do histology on them. Those are real challenges.” This has propelled Organovo as well as many other companies and research teams to set their sights on 3-D human tissue for both drug discovery research and therapeutic applications.
One of the teams making considerable progress in moving the field from 2-D to 3-D is that of Matthias Lütolf, Ph.D., a professor professor with the Institute of Bioengineering at École Polytechnique Fédérale de Lausanne (EPFL) in Switzerland. Last year, Lütolf and his doctoral student Nathalie Brandenberg (Figure 2) announced that they had developed a way to direct cells and developing tissue in three dimensions using a combination of photoablation and microfluidics [1].

“Conventionally, we use microdevices to grow cells and to try to generate tissues— this is common with work on organs-on-a-chip or labs-on-a-chip—but when we do this, we encounter difficulties, because the cells are growing on substrates that are not very cell friendly or not very physiological: they are sitting on a piece of plastic or glass. This is not really an environment that would allow you to build tissues,” Lütolf observes.
What may be needed to generate more lifelike tissues are precise biochemical and physical “instructions” delivered to cells at the right time and location in three dimensions, as well as with spatial boundaries, he says. Such boundaries can guide the self-organization of the growing cells into tissues within the same kinds of 3-D spaces that physically constrain—as well as physiologically influence— their biological counterparts.
To introduce the spatial constraints and create a means to deliver important cell signaling factors in 3-D cultures, Lütolf and Brandenberg employed photoablation using focalized short-pulse lasers to carve microfluidic networks into biocompatible hydrogels (Figure 3). “This way, we create a connected network of channels that can be perfused with biomolecules or filled with cells,” he explains. The technique allows them to influence the growth and organization of the cells and the shape of a new tissue (e.g., a blood vessel). Because the technique can be performed “in very close proximity to living cells,” it can also be performed as the cells multiply to control subsequent tissue growth, Lütolf says.
To develop the approach, Brandenberg and Lütolf refined a laser dissection microscope by adding precision laser positioning and building software to program the laser and generate what can be quite complex ablation patterns. Next, they constructed an interface to create a functioning microfluidic system. “The rest,” he continues, “was a lot of characterization of the method—how much intensity you need, what types of gels can be ablated, what is the effect on the cells that are sitting close to where you etch away material—to be sure that this is reliable and reproducible.”
Now that they have figured out how to make channel networks, Lütolf sees a wide range of possible applications, including in stem cell-based organoids, which are miniature, 3-D, lab-grown structures that resemble an organ. A current hurdle with organoids is that they grow in “very uncontrollable ways,” Lütolf says. “The reason is that cells need certain factors to grow, and these factors must be delivered in very precise ways in space and time. In other words, only one part of a growing tissue might see this particular factor [called a morphogen] at this time, and another part might see a different factor at another time. When we flood cells in culture with a homogeneous concentration of a factor, there’s no surprise that the cells are really lacking the instruction to build tissue. That’s what’s been happening.”
With their photoablation technique, Lütolf believes they may address this problem. “The idea is to carve out a channel in close proximity to a growing structure and, at some point in time, go in and deliver a pulse of a key molecule just very locally, and that should allow more control over how cells, as well as stem cells, self-organize,” he says. His group is currently growing organoids in the lab and using this microfluidic delivery of morphogens to control tissue development.
Overall, Lütolf anticipates his lab’s contributions to generating channel networks will facilitate the work of other research labs. “With our technique, we hope we make tissue engineering studies easier and more accessible, especially to biology labs that want to do these types of experiments.”
Feeding the Tissues

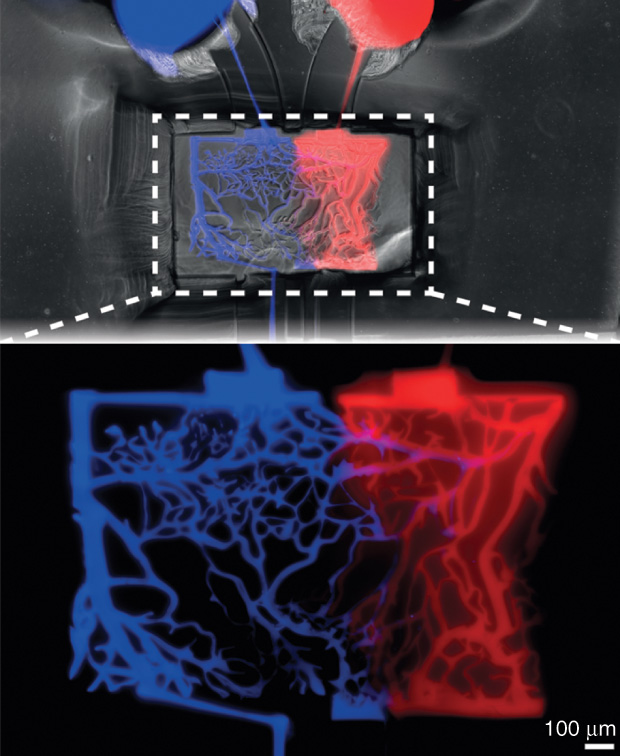
One of the major interests in channel networks is their use in vascularization, or in bringing oxygen and other nutrients to the engineered tissue. Without vascularization, labs could only make engineered tissues that are no more than a half of millimeter thick. Anything higher, and the cells within the tissue would starve to death. In 2016, however, the research group of Jennifer Lewis, Sc.D., Hansjörg Wyss Professor of Biologically Inspired Engineering at Harvard University’s School of Engineering and Applied Sciences (Figure 4, right) announced that it had developed a multimaterial bioprinting method that could introduce vascularized networks capable of sustaining three-dimensional engineered tissues that were a full centimeter thick.
The work builds on previous bioprinting advances made in Lewis’ lab. Three years ago, Lewis and her group demonstrated a multimaterial bioprinting process that enabled them to print the extracellular matrix using hydrogel [2]. This provided structural support for the growing tissue while simultaneously incorporating vascular channels into it. They accomplished the feat by developing a disappearing ink (they call it “fugitive ink”), which is a polymer solution that melts away at low temperatures. As a result, they can draw intricate patterns such as branching networks within the hydrogel, chill the whole structure to 4 °C to melt away the ink, and leave behind now-empty channels throughout the hydrogel matrix.
With an open vascular network now at the ready, the group then floods the channels with a solution that contains endothelial cells (like those lining blood vessels in living tissue). “The cells adsorb onto the tissue interface and form a cell layer, so we’ve created a vascularized, 3-D tissue. More recently, we have been able to show the ability to construct a tissue that is about a centimeter thick and with a heavily pervasive vascular network,” Lewis says [3].
In addition, Lewis and her group demonstrated that they could utilize human stem cells, specifically mesenchymal stem cells, and perfuse the tissue architecture with cell media that would drive those stem cells down a specific lineage. “In this case, we induced bone mineralization. That has been our most sophisticated result to date, because it’s a true thick tissue in which we were able to drive stem cells toward a specific tissue architecture,” she explains.
In addition, her group worked with the Roche Innovation Center in Basel, Switzerland, to generate channels they lined with different epithelial cells, particularly the epithelial cells present on the inner periphery of proximal tubules in the kidney, and were able to produce a working kidney-specific unit [4]. “That project was important because we showed that the three-dimensionality of that structure really made a difference to the cell function and morphology, allowing us to better recapitulate a human tissue in the native environment,” Lewis notes. She quickly points out that it doesn’t mean they have made a completely functional kidney. “This is a starting point. For our group, we want to first tackle ex vivo, outside-of-the-body, applications, such as drug screening and disease models.”
While great progress has been made, much is left to do, she remarks. “It’s not enough to just put down cells in a 3-D construct and keep them alive via perfusion through their embedded vasculature; you ultimately want function. We’re not completely there yet,” she says, adding that she would also like to create instrumented tissues with embedded sensors. “I think the possibilities are stunning, but I also think the challenges are significant, as we have to make sure the implanted tissue functions like native tissue and is not rejected by the body. Those are things that the field continues to wrestle with.”
Form and Function
One company tapping into the possibilities of tissue engineering is Organovo [5], which is using its proprietary bioprinting technology to generate 3-D human tissue models for drug discovery and other purposes. As have other research groups, the company is finding that when it comes to tissue engineering, less is not more. In fact, more is far superior.
“There’s this drive when testing drugs preclinically to see if we can get very complicated biology boiled down to as simple a readout as possible—one protein, one cell type, one enzyme,” says Organovo’s Nguyen. While the simplicity sounds good, it doesn’t work especially well for this application. “Humans are complex creatures with very complex biology. We make decisions and we pick drugs based on those simple readouts, and then we end up being surprised that they don’t work when we put them into people. But, in truth, we shouldn’t really be surprised.”
This appreciation has led tissue-engineering researchers to embrace the complexity rather than seeking to sidestep it. At Organovo, that means bioprinting detailed 3-D tissue structures that approximate living tissue as closely as possible. “We’ve found that the function really follows the form,” Nguyen says.
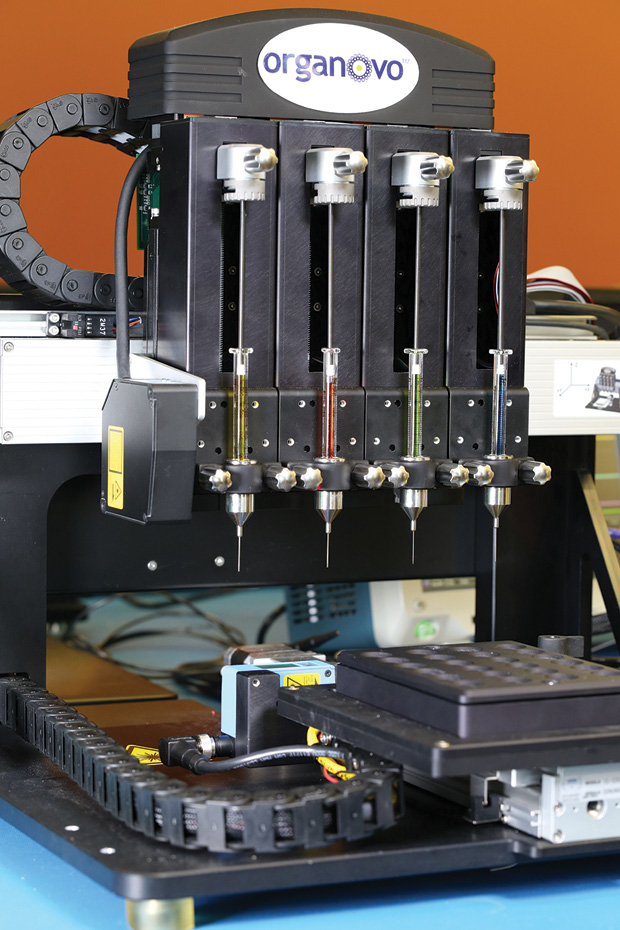
The company’s NovoGen Bioprinter (Figure 5) has evolved over the past decade from initial work done at the University of Missouri–Columbia. Rather than seeding cells within hydrogels or onto scaffolding, the platform takes a different approach by laying down cell-laden materials one layer at a time to build the tissue (Figure 6). This yields a highly organized structure that closely mirrors native tissue, and it can produce tissues 500-μm thick without central cell necrosis, Nguyen explains. “With these structures, we can get more clinically relevant analyses than you can with smaller engineered tissues. For example, the bigger pieces of tissue allow us to do more analyses, so we can have enough of the media [in which the tissues are cultured] to do analyses for biomarkers that might translate to patients, or to get out enough RNA to do wholegenome analysis and test for the impact of chemical exposure.”

To illustrate the usefulness of the 3-D platform, Nguyen described two of the company’s recently launched products: bioprinted liver tissue and bioprinted kidney tissue [6]. With both, Organovo is working with pharmaceutical customers who are using the tissues to evaluate the toxicities of molecules and compounds for therapeutic purposes. Such a vetting process is very efficient, because it can eliminate poor options early on and long before human trials. “Our customers are able to make better decisions in their drug-development pipelines based on the data we’re able to provide them using these tissues,” she notes.
Organovo is also getting involved on the therapeutic side and in December presented data showing that it was able to transplant functional, 3-D, bioprinted human liver tissue into animal models. According to the company, the preclinical data revealed “evidence of stable production of key human liver proteins in the animal bloodstream, and tissue staining for key human metabolic enzymes [that provide] an important first step in demonstrating the capability of this tissue to treat inborn errors of metabolism, a key indication we are targeting.” Based on these results, Organovo is continuing preclinical development of the tissue for therapeutic use.
In addition, the company is expanding its 3-D kidney efforts through a collaboration with Prof. Melissa Little, who heads the Kidney Research Laboratory at the Murdoch Children’s Research Institute of The Royal Children’s Hospital in Melbourne, Australia, and is also a professor in the University of Melbourne’s Faculty of Medicine, Dentistry, and Health Sciences. “Our initial kidney product at Organovo focuses on a very narrow region of the kidney, called the proximal tubule, and while that site experiences a lot of potential side effects and toxicity in the body, it’s just one small section of a functional kidney,” Nguyen remarks. “Dr. Little, on the other hand, has developed fascinating technology in which she starts with stem cells and is able to create more regions of an intact nephron, which is the functional unit of a kidney, in a dish.” The ultimate goal, according to Nguyen, is to combine Little’s expertise with Organovo’s bioprinting technology and possibly impact the therapeutic side. “She will be working with one of our bioprinters in her laboratory to try to make that a reality.”
Into the Clinic
As progress toward larger, vascularized, and functional engineered tissues continues, RenovaCare Inc. of New York City is taking a different tack and getting right into regenerative medicine [7]. Specifically, the company is designing an advanced technology, its trademarked SkinGun, that sprays a patient’s own stem cells onto damaged skin, with the goal of repairing tissue in a far less arduous way than that afforded by current methods.

“Traditional skin grafting involves the removal of large sheets of healthy skin from a patient, the puncturing of each sheet in a grid-like pattern to form an expandable mesh, the stretching of each sheet as far as feasible, and then its stitching onto the patient’s wound,” says Thomas Bold, president and CEO of Renova-Care (Figure 7). “It not only sounds bad, but it is also extremely painful and creates an additional wound at each donor site. The results are very often poor, with resulting scarring and deformed skin [and] limited joint movement, and, if the patient is young, the skin has the inability to grow with the patient, especially in young children. Long-term pain management, months and sometimes years of physical therapy, and oftentimes additional surgeries are needed after the initial treatments.”
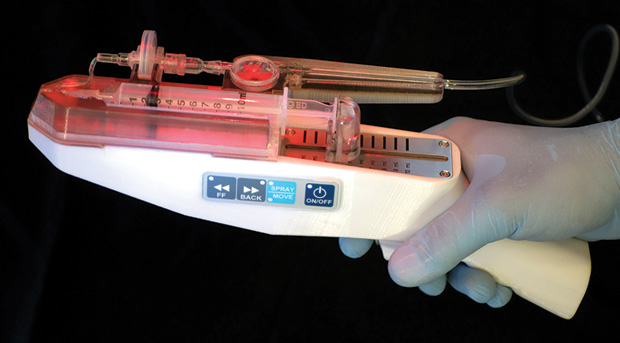
RenovaCare has received a number of patents for its technology, including one from the U.S. Food and Drug Administration (FDA) in December 2016 for its SkinGun device that sprays a mist of the patient’s stem cells onto the wound site (Figure 8). “Normally, when you have a wound, it heals from the edge to the middle, and the longer this process takes—and the larger the wound is—the higher the risk for inflammation, infection, scarring, long-term pain management, and long-term physical therapy. With our system, we’re spraying on thousands and thousands of little regenerating islands all over the wound, and, as they grow, they connect to each other, finally closing the wound with an epithelial layer,” Bold explains (Figure 9).
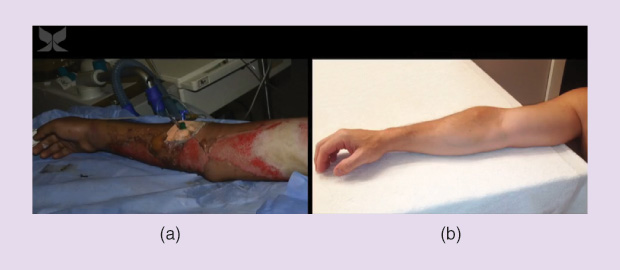
This newly formed layer is pink and delicate, but it is dry and provides a sufficient level of protection that the patient can leave the hospital. “The patient doesn’t have to come back for more treatments, because the skin will continue to naturally and cosmetically heal,” he says. “The skin that regrows looks, feels, and functions as the original skin that it replaces.”
To develop the technology, researchers designed an isolation protocol that can quickly free the stem cells from the surrounding tissue and then achieve high cell viability (tested at 97.3%) even as the cells are sprayed onto the wound, according to Bold. In addition, the donor contribution is quite small. “A very important note here is that while the sheet of meshed skin [used in traditional skin grafts] covers only about six times the original donor area, with the RenovaCare system we can cover up to 100 times the size of the donor skin sample, so the sample can be very small compared to the injury-treatment site,” he explains.
The SkinGun has already been used successfully on a limited number of human patients to treat second-degree burns and is undergoing additional testing. “We still have to go through the regulatory process with the FDA, but technologically we are there—the technological development is finalized and we have a working system,” Bold says.
While the SkinGun was originally developed to treat burns, the company is pursuing other potential applications. “We see our technology as feasible for additional indications, such as pigmentation disorders, scar removal, cosmetic treatments, or possibly for other diseases,” Bold elaborates. “There are all kinds of options for the future of this technology, even expanding into other possibilities for using the body’s capacity to heal itself. On the one hand, the most simple thing you can imagine is using your own body to heal itself, but on the other hand, we are just at the beginning of that potentiality. As we look into the future, this will be the medicine of the future.”
Off to a Good Start
The prospects for tissue engineering are indeed mind boggling, and research groups around the world are toiling away to bring them into sharper focus. “From my point of view,” says Nguyen, “I think one of the big things that people are really going to be concentrating on for the next five to ten years is to incorporate aspects of fluid flow into 1) the in vitro systems to see how close to native tissue and biological functionality we can get and 2) into therapeutic tissues, because you need perfusable vasculature for larger-scale tissue structures—the more tissue you can create to help replace function, the more patients you can treat and the more of an impact you can have.”
To meet that vasculature challenge, different approaches and technologies will have to come together, she continues. “Look at just bioprinting. We talk about bioprinting as one thing, but it’s really a variety of different technologies that are brought to bear to create a 3-D structure. Most laboratories still stick to just one technology, perhaps extrusion bioprinting or laser-assisted bioprinting, or maybe only inkjet types of deposition.” To make the leap to large tissue structures, however, “we’re going to need platforms that allow you to have many different types of fabrication operated together to create both structures with fine detail, and also with the bulk and heft that you’re going to need in a real replacement application. And we need people who are specialists in their areas but who are working as part of broader collaborations that can put all of these pieces together,” Nguyen predicts. “It’s really going to be a cross-disciplinary effort.”
Lütolf is beginning to see just that type of collaborative effort. “I’ve been in this field since I started as a young Ph.D. student many years ago, and in the last few years I have seen a convergence of fields like never before. There is really a multidisciplinary effort now that is not only driven by the clinicians or the engineers but also by the best developmental biologists and stem cell biologists, which in my opinion is something that has always been missing in the field and is needed.”
As this collaborative effort is now combining with technologies that have matured tremendously on all levels, including very sophisticated chemistry, materials, and microtechnology, Lütolf believes everything is falling right in line. “Of course, people said the same thing ten years ago, but what I’m seeing now is different. All the ingredients are now in place to have a huge impact on the field.” He adds, “I am seeing a true convergence that will lead to very exciting advancements in tissue engineering over the next few years.”
References
- N. Brandenberg and M. P. Lütolf, “In situ patterning of microfluidic networks in 3D cell-laden hydrogels,” Advanced Materials, vol. 28, no. 34, pp. 7450–7456, 2016.
- D. B. Kolesky, R. L. Truby, A. S. Gladman, T. A. Busbee, K. A. Homan, and J. A. Lewis, “3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs,” Advanced Materials, vol. 26, pp. 3124–3130, 2014.
- D. B. Kolesky, K. A. Homan, M. A. Skylar-Scott, and J. A. Lewis, “3D bioprinting of thick vascularized tissues,” PNAS, vol. 113, no. 12, pp. 3179–3184, 2016.
- K. A. Homan, D. B. Kolesky, M. A. Skylar-Scott, J. Herrmann, H. Obuobi, A. Moisan, and J. A. Lewis. (2016, Oct. 11). Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Scientific Reports. [Online]. vol. 6, p. 34845.
- Changing the shape of medical research and practice. [Online].
- S. M. King, J. W. Higgins, C. R. Nino, T. R. Smith, E. H. Paffenroth, C. E. Fairbairn, A. Docuyanan, V. D. Shah, A. E. Chen, S. C. Presnell, and D. G. Nguyen, “3D proximal tubule tissues recapitulate key aspects of renal physiology to enable nephrotoxicity testing,” Frontiers Physiol., vol. 8, p. 123, Mar. 2017.
- Spray-on stem cells for rapid healing. [Online].



