Floating in a Petri dish, they look like tiny tapioca pearls in peach broth, a couple dozen in number and none much larger than the tip of a ballpoint pen. But under a microscope, dense, lumpy bodies come into focus, outlined by wispy coronas.
“They’re forming the cerebral cortex,” says Li-Huei Tsai, director of Massachusetts Institute of Technology’s Picower Institute for Learning and Memory, gesturing at the screen. That pale corona, she explains, is actually the beginning of the neuroepithelial layer, one of the earliest steps in the formation of a human brain.
Of course, these aren’t real brains she’s growing in her laboratory incubator. They’re two-and-a-half-month-old cerebral organoids—colloquially, minibrains—grown, in this case, from the skin cells of adult subjects who carry a genetic risk factor for Alzheimer’s disease. If all goes according to plan over the next few months, the organoids should become more complex, developing clusters of neurons and a three-dimensional (3-D) structure reminiscent of the developing human cortex.
Cerebral organoids make no attempt to replicate true brains, but they do represent a more complex, brain-like model than researchers have ever had before. This is crucial because many diseases of the brain are too complex to be accurately represented in a flat culture, where the circuitry and interplay of cell types in 3-D space are impossible. Even something as simple as modeling basic Alzheimer’s pathology doesn’t work well in such cultures because the signature protein tangles diffuse into the media. Similarly, mouse models, as useful as they are for behavioral studies, have cells that operate on different schedules, lack an elaborate neocortex, and don’t spontaneously develop human conditions like autism, schizophrenia, and microcephaly. Organoids not only have the potential to break these limits—in some cases, they already have.
Cells in the Know
The minibrain systems grew from a convergence of insights over the last decades. In 2006, scientists determined how to take human cells of any kind and reprogram them into pluripotent stem cells— sidestepping embryonic issues and allowing labs to create stem-cell lines preprogrammed with any individual’s full genetic information. These cells, like embryonic stem cells before them, also knew what to do; with a minimum of guidance, for example, induced stem cells can spontaneously clump together and differentiate into self-organized rosettes. Using both these and embryonic stem-cell lines, researchers began coaxing neural cultures into larger and more complex bodies. These efforts culminated in 2011 with the first 3-D model—retinal tissue creating optic cups—from the lab of the late, preeminent Japanese stem-cell biologist Yoshiki Sasai. Within two years, his lab had gone on to use a similar protocol of floating cultures to produce an elaborate forebrain-like structure of neurons and glia with a curving shape, rudimentary layers, and key developmental zones reminiscent of early fetal brain development. He showed later that the method also worked with other parts of the brain, such as the cerebellum and hippocampus.
In the same year that Sasai reported his forebrain discovery, Juergen Knoblich and Madeline Lancaster at the Austrian Academy of Science independently discovered a way to embed clumps of neural stem cells into droplets of a protein matrix—Matrigel—and a spinning bioreactor to produce whole-brain organoids with signs of hind-, mid-, and forebrain regions, in addition to neurons and glial subtypes.
A rapid series of improvements followed in the wake of these reports. A team led out of Johns Hopkins University tapped 3-D printing to develop cheaper, scalable versions of Lancaster’s spinning bioreactors. At the Whitehead Institute in Massachusetts, researchers collaborating with Lancaster and Knoblich identified gene manipulations that would produce a semblance of the signature grooves and bulges of the human brain, while a group at Stanford University published their success in producing a simpler 3-D neural model so dense with such active synapses that the researchers could even perform electrophysiological recordings.
Model Z
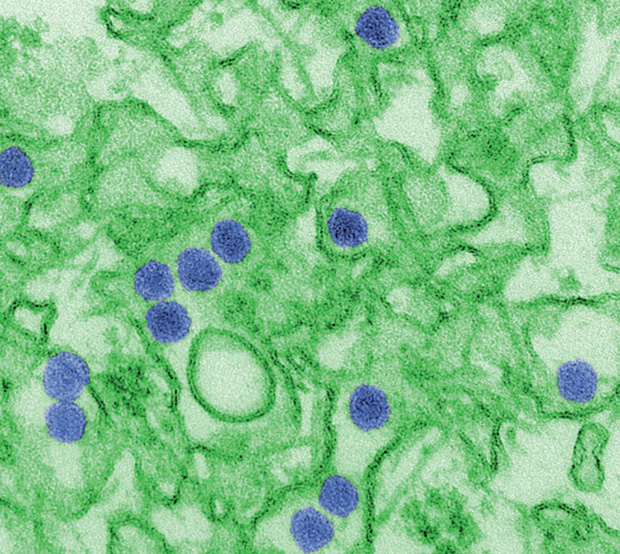
Today, these minibrains are only a few years old and still far from perfected. Yet they have already begun to be practically applied to high-profile issues like the Zika infection (Figure 1), Alzheimer’s, and autism, with surprisingly powerful effects.
In Tsai’s first published attempt with the technology, appearing last year in the journal PLoS One, she showed that organoids grown from the skin of Alzheimer’s patients spontaneously demonstrated both of the disease’s hallmarks—tau tangles and amyloid plaques, elusive in monoculture—and responded to known anti-amyloid drug interventions.
“If you want to produce a system that displays full-blown Alzheimer’s pathology and symptoms, I would say, it doesn’t exist outside of human patients,” Tsai says. “But if you just want to use the cells to model a certain pathology, with the original mutation and the same genetic background, I cannot think of any better system.”
The very first application of these types of organoids in neurological disease appeared in 2013. Part of Lancaster and Knoblich’s protocol debut included a proof-of-concept model of microcephaly—a particularly difficult condition to recreate in mice. Organoids produced from the skin of a microcephalic patient were not only stunted compared to controls, but the researchers were also able to delve into the diseased tissue and discover why that might be. The organization of the dividing cells was askew, they noticed, compared to controls, and the brains were producing neurons ahead of schedule instead of continuing to proliferate more, and more varied, cell types.
The key gene mutation carried by the patient in that study and shown to be causally linked to the organoids’ growth wasn’t breaking news; it had already been suspected by researchers. But the success of the microcephaly model catapulted it into the spotlight two years later, when the Zika virus made headlines.
At that time, Stevens Rehen, with the D’Or Institute for Research and Education in Rio de Janeiro, Brazil, had just begun growing organoids in his lab based on Lancaster’s model, hoping to develop studies of mental disorders like schizophrenia (Figure 2). When the news broke, he immediately redirected his lab’s effort into Zika instead, and by April 2016 he had published the first report showing that the virus definitively stunted organoid growth.
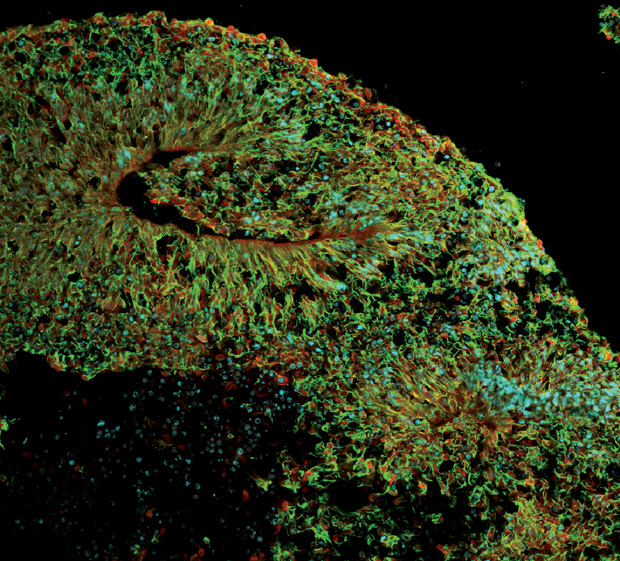
He has since followed that work with another publication in collaboration with colleagues from Rio’s Oswaldo Cruz Foundation, demonstrating how the hepatitis C drug sofosbuvir can block Zika replication. The drug was chosen because it is both already used in pregnant women and targets a protein conserved among the virus family that includes both hepatitis and Zika. That paper used several models, including simple stem-cell lines in addition to the organoids. The latter, he explains, were what really allowed him to test the drug’s protective ability on something much closer to the complexity of a real brain. “These models with this 3-D culture—they are awesome and very important,” Rehen notes. “It helps us to understand the consequence of the infection.”
Zika has become a widely used disease to trial in minibrains, due in part to the urgency of the outbreak and the easily measurable brain abnormality—microcephaly—that it can cause. Last May, researchers at the University of California, San Francisco, led by stem-cell biologist Arnold Kriegstein, used tissue samples and organoids to help demonstrate how a certain protein expressed in radial glial cells—a type of neural cell important to cortical growth and prominent in organoids and fetal tissue— might be key to the virus’s infection. In addition, both the Whitehead Institute team that discovered how to produce cortical folds in organoids and the Johns Hopkins team behind the printable bioreactors used the Zika virus as proofs-of-concepts in their publications. “My editor wanted to have a disease model for the system,” says Johns Hopkins neuroscientist Guo-Li Ming, senior author on the paper. “And I talked to my graduate student, who happened to be reading the news about Zika on his computer, and he said, well, how about Zika?”
Researchers anticipate that organoids will be able to advance work in other mental diseases as well. In 2015, Yale neurobiologist Flora Vaccarino successfully trialed organoid models using cells made from autistic patients. She discovered how increased activity of a certain gene seemed to be responsible for creating excessive numbers of inhibitory gamma-aminobutyric acid (or GABA) neurons, throwing off the balance of excitation and inhibition in the developing minibrains. Both Ming and Rehen have begun experimenting with organoid models of schizophrenia. In earlier work, Ming explains, her lab had already found slower synaptic development in traditional cultures from schizophrenic patients, and they anticipate being able to find even more in 3-D views—”because now we’ll be able to see not just the neuronal populations but other cell types,” she explains, “maybe to see whether there are even earlier phenotypes affecting development here.”
Challenges Still Abound
“If you look at the broader picture, it really is almost miraculous that you can start with a skin cell from an adult patient, convert it into a stem cell, tweak that cell in a certain way to get it to start behaving like an embryo, then again to become a nervous system, and then eventually develop into something that resembles the early human developing brain. It’s just amazing,” says Kriegstein.
“It’s when you get into the weeds,” he adds, “and start looking more carefully at what’s being produced [that] you see all the problems.”
The biggest problem, in Kriegstein’s view, is the variability that still haunts current minibrain cultures. Even when grown at the same time under the same conditions or produced from the same line months apart, he says, organoids can be very different, and these differences are not always obvious. For example, two cortical organoids might look superficially alike; but, genetically, one might be more like the medial cortex, which includes important signaling areas, while the other might lack the signaling region and look much more dorsolateral. Whole-brain, intrinsic protocols like Lancaster’s are particularly prone to these issues, though more targeted approaches like Sasai’s are still not wholly immune.
Researchers are already exploring various strategies to solve the issue. In her autism paper, Vaccarino added an extra step to the process, which was already based on Sasai’s protocol, by manually selecting cells already heading toward a forebrain fate while her stem cells were still in the first stages of differentiation. Lancaster and Knoblich published a report on the preprint repository bioRxiv last spring about their success in producing more organized and less variable products by seeding polymer microfilaments with stem cells prior to embedding them in Matrigel and setting them to spin.
One of the biggest rate-limited steps of all is the lack of blood vessels. It’s the same problem that plagues engineered tissues across the board. Without a way to pump blood throughout the cultured bodies, organoids tend to die at a certain point. This limits how far these systems can be pushed.
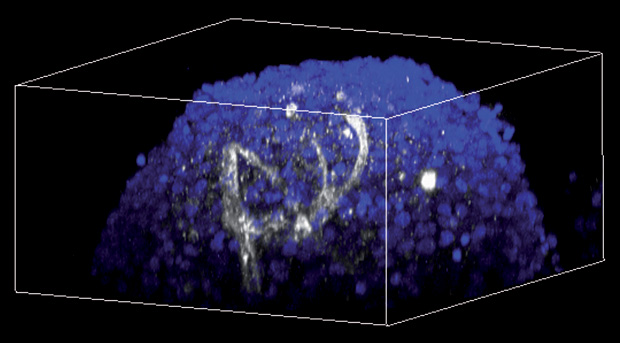
There are tantalizing hints of ways to solve the issue. At Brown University earlier this year, bioengineer Diane Hoffman- Kim reported the surprising discovery of tiny networks of hollow, capillary-like tubes that had spontaneously appeared in neurospheroids her lab had been exploring for other purposes (Figure 3). Spheroids are a simpler model than organoids, and the cells were grown from neonatal brain tissue— significantly different from lab-made human stem cells. But she is already in the process of trying to replicate the effect in human, induced human stem cell neurospheroids and has plans to experiment with microfluidic systems that could pump fluid through potential capillaries. “We’ve been sketching on napkins for about a year now,” she says, “but I actually think we have a real design now.”
Lancaster, who is now at the Medical Research Council Laboratory of Molecular Biology in England, predicts a significant role for bioengineers to help with these kinds of challenges. Indeed, she is coorganizing a 2018 workshop with the Company of Biologists publishing group to promote exactly such a collaboration. “I can say that there are a number of partnerships that I know of now ongoing, and I think in the next year or two we will see some exciting new approaches,” she says.
Tsai is also studying new ways to engineer specificity and blood vessels into her 3-D systems. “Of course, every system has its limitations,” she says. “But you just try to take advantage of what it can offer.” She envisions a day when researchers like herself will have developed techniques to mix different cell types together in complex, reproducible models, fit with primitive vasculature—maybe even a way to pump liquid through them like an artificial heartbeat. “It’s our dream,” she says. “You can dream first and then see how far you can go.”
For Further Reading
- P. P. Garcez, E. C. Loiola, R. Madeiro da Costa, L. M. Higa, P. Trindade, R. Delvecchio, J. M. Nascimento, R. Brindeiro, A. Tanuri, and S. K. Rehen, “Zika virus impairs growth in human neurospheres and brain organoids,” Science, vol. 352, no. 6287, pp. 816–818, May 2016.
- T. Kadoshima, H. Sakaguchi, T. Nakano,M. Soen, S. Ando,M. Eiraku, and Y. Sasai, “Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell–derived neocortex,” Proc. Nat. Acad. Sci. USA, vol. 110, no. 50, pp. 20284–20289, Dec. 2013.
- I. Kelava and M. A. Lancaster, “Stem cell models of human brain development,” Cell Stem Cell, vol. 18, no. 6, pp. 736–748, June 2016.
- M. A. Lancaster, M. Renner, C. A. Martin, D. Wenzel, L. S. Bicknell, M. E. Hurles, T. Homfray, J. M. Penninger, A. P. Jackson, and J. A. Knoblich, “Cerebral organoids model human brain development and microcephaly,” Nature, vol. 501, no. 7467, pp. 373–379, Sept. 2013.
- J. Mariani, G. Coppola, P. Zhang, A. Abyzov, L. Provini, L. Tomasini, M. Amenduni, A. Szekely, D. Palejev, M. Wilson, M. Gerstein, E. L. Grigorenko, K. Chawarska, K. A. Pelphrey, J. R. Howe, and F. M. Vaccarino, “FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders,” Cell, vol. 162, no. 2, pp. 375–390, July 2015.
- W. K. Raja, A. E. Mungenast, Y. T. Lin, T. Ko, F. Abdurrob, J. Seo, and L. H. Tsai, “Self-organizing 3-D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes,” PLoS One, vol. 11, no. 9, pp. e0161969, Sept. 2016.



