The human gut is home to an abundant and diverse community of microbes—each of us carries roughly 100 trillion, representing more than 1,000 different species. The composition of one’s gut microbiota is individually specific, dynamic, and influenced by genetics, diet, age, metabolism, medication use, stress, and geography. These bacteria perform a range of necessary and beneficial functions, including breaking down our food and supporting our immune systems.
But mounting evidence suggests that immunity and digestion are just a small part of what all of those microbes actually accomplish. Researchers are finding that microbes seem to play a role not only in intestinal health but also in psychiatric health, including an individual’s proclivity to depression. “We see it as a psychobiotic revolution,” says John Cryan, a neuroscientist at University College Cork (Figure 1). “Psychobiotics, or nutritional psychiatry, is a whole new way of tackling mental health issues— through the gut bacteria.”

How the Gut and the Brain Communicate
Humans have a second brain, called the enteric nervous system, that consists of approximately 100 million neurons embedded in the walls of our gut. The enteric nervous system is one player in the gut–microbiome–brain axis (Figure 2), a complex two-way communication system between our gut microbes and our brains. Scientists have not worked out the nitty-gritty mechanisms that link specific microbes to the brain, but there are a number of potential pathways.
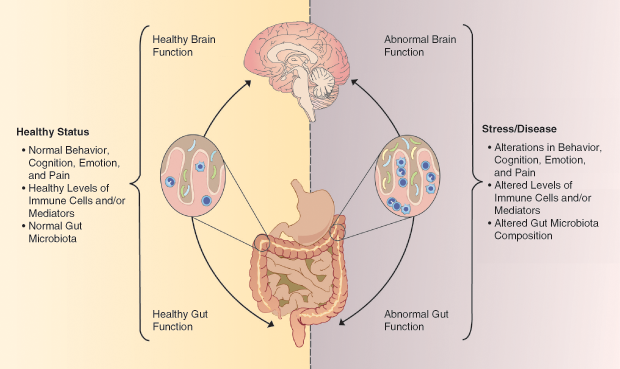
One way that gut microbes might communicate with the central nervous system is through the production of neurotransmitters and other neuroactive chemicals. More than 90% of the body’s serotonin, for instance, is found in the gut. Serotonin helps regulate gastrointestinal (GI) motility, but it also has a well-known role in mood disorders like depression. In addition to directly producing neurotransmitters, gut bacteria can also affect how people metabolize these compounds, influencing the amount that reaches the brain. Although the enteric nervous system makes use of more than thirty neurotransmitters, including serotonin and dopamine, the details of how these chemicals act locally on the gut or how they might influence the central nervous system are not well understood.
A second probable pathway for the microbiome–gut–brain axis is the vagus nerve, a main conduit between the brain and the gut. Cryan and others have shown in animal studies that some microbes can activate this nerve.
Finally, the gut microbiome is intertwined with the immune system. Bacterial products act on the immune system, for instance, via circulating levels of immune molecules such as cytokines. Elevated blood levels of inflammatory cytokines have been associated with depression, and their levels are partially regulated by gut microbiota.
The bidirectional nature of gut–brain communication is illustrated by the high degree of overlap between GI and psychiatric disorders. Patients with GI disorders have higher rates of anxiety and depression than the general population, and patients with anxiety and depression experience more GI symptoms than healthy people.
Learning From Microbe-Free Mice
Major scientific interest in the microbiome–gut–brain axis was sparked by a 2004 study of germ-free (GF) mice. GF mice are bred and kept in sterile isolation chambers. Because they completely lack gut microbes, scientists can use them to directly assess the contribution of gut bacteria to depression-like symptoms or changes in brain development and physiology.
For their study, researchers at Kyushu University in Japan exposed GF and normal mice to stress by temporarily restraining them in narrow tubes. They found that, in response to stress, the animals lacking gut microbes produced far more stress hormones than the normal mice. In addition, pretreatment with a single microbe (a bacterium called Bifidobacterium infantis) reduced the overactive stress response of GF mice.

These results showed for the first time that gut microbes could influence stress responses in the brain and suggested that probiotics could be a potential treatment for psychiatric disorders. However, the idea that intestinal microbes influence the mind was met with skepticism for several years.
Jane Foster, a neuroscientist at McMaster University in Ontario, Canada, recalls how difficult it was to publish her first paper on GF mice and behavior (Figure 3, right). “Our initial study showed changes in anxiety-like behaviors in GF mice,” says Foster. “We had those data for a long time before anyone was willing to publish them. We conducted those studies in 2005, but the paper wasn’t published until 2011. We submitted it a million times.”
Stress, Bacteria, and Depression
Despite initial skepticism, research has continued to demonstrate a link between gut bacteria, the brain, and depression. In a recent study, Premysl Bercik, a gastroenterologist at McMaster University, showed how early life stress figures into that equation. He and his colleagues demonstrated that stress in mice shortly after birth, together with the gut microbiota profile associated with stress, can lead to depression later in life.
Early life stress is a predisposing factor for both depression and some functional GI disorders, such as irritable bowel syndrome. Bercik and his colleagues stressed GF and normal mouse pups by separating them from their mothers for several hours a day over two weeks. The baby mice with normal gut microbes that experienced early life stress exhibited signs of depression and anxiety, and they produced more of the stress hormone corticosterone. The GF mice, on the other hand, showed the same increased stress hormone levels, but they did not show any signs of anxiety or depression.
Then, Bercik and his team transferred bacteria from stressed mice into GF mice. Within several weeks, the mice started showing signs of anxiety and depression. Bercik says that the results suggest that it is not only gut bacteria but also changes in the microbiome–gut–brain axis that lead to depression in animals subjected to early life stress. “The study showed that early life stress leads to increased stress reactions and gut dysfunction that changes the gut microbiota, which in turn influences behavior.”
Gut-Centric Treatment
The ability of gut microbes to regulate brain function and impact behavior suggests the potential for new mood disorder therapeutics. There is some preclinical evidence that the manipulation of gut microbiota with specific probiotics can influence depression-like behaviors in animal models. Cryan has demonstrated that strains of two bacteria, Lactobacillus and Bifidobacterium, seem to be key players in the microbiome–gut–brain axis. Cryan and his colleagues found that the administration of either of these two bacteria was as effective as the antidepressant Lexapro in treating depression symptoms in mice.
Yet, despite compelling animal evidence, there is little research on the microbiome–gut–brain axis in humans. Of the limited work in humans that has been published, there is some support for the potential use of probiotics to treat depression. For example, Bercik and his colleagues treated patients exhibiting concomitant irritable bowel syndrome and depression with a specific probiotic that improves depressive behavior in mice. After six weeks of treatment, patients who received the probiotic scored lower on tests of depression. What’s more, their brain activity patterns, measured with functional magnetic resonance imaging (fMRI), had changed as well (see also the article “Imaging Depression” on page 19 of this issue). “This probiotic decreased reactivity to emotional stimuli in a network of brain centers that are involved in mood regulation and are also targeted by antidepressants,” says Bercik. “It’s one of the first randomized, controlled clinical trials that shows treatment with a specific probiotic can affect mood and brain activity.”
Another well-known study was conducted by Emeran Mayer, codirector of the Digestive Disease Research Center with the David Geffen School of Medicine at the University of California, Los Angeles, and author of the book, The Mind–Gut Connection: How the Hidden Conversation Within Our Bodies Impacts Our Mood, Our Choices, and Our Overall Health.
In this study, healthy women ate a cup of commercially available yogurt twice a day for four weeks. Yogurt contains live bacteria: in this case, strains of Bifidobacterium, Streptococcus, Lactococcus, and Lactobacillus. Mayer and his colleagues found differences between the women who ate yogurt and a control group who did not when the two were examined by fMRI. While completing an emotional recognition task, the probiotic group showed changes in midbrain connectivity. Mayer thinks that the bacteria in the yogurt changed the composition of the participants’ gut microbes and this resulted in changes in brain chemistry.
A Diet to Fight Depression
Mayer’s study offers intriguing evidence that probiotics ingested in food can alter human brain function. But can mood disorders really be prevented or treated with dietary changes?
It is known that poor diets, such as those that consist primarily of high-fat, sugary foods, pose a risk factor for depression. Poor diets may perpetuate changes in gut microorganisms and drive depression symptoms, while healthy diets may prevent depression. Dietary changes could be prescribed for patients, particularly those with milder symptoms of depression, as an alternative to drug therapy with unwanted side effects.
To keep one’s gut healthy, Mayer recommends eating a predominantly plant-based diet with no or very little red meat. “The high plant content provides food for your microbes, which will increase their abundance and diversity and minimize gut immune activation,” he advises.
Other recommendations include eating complex carbohydrates and fermented foods like kimchi, kefir, and yogurt. Foods to avoid include artificial sweeteners, emulsifiers, and highly processed foods, all of which have been shown to negatively impact the gut microbiome.
Fecal Transplants For a Better Mood
In addition to probiotics and dietary changes, scientists are looking to fecal microbiota transplants as a treatment option. Fecal microbiota transplantation is being tested as a method to manipulate or modulate the gut microbiome in a way that may relieve diseases like depression associated with abnormalities in the microbial community of the gut.
In a study by Cryan and colleagues, a fecal microbiota transplantation from depressed human patients to GF rats resulted in behavioral and physiological symptoms of depression in the animals. “In effect, we were able to transfer the blues,” says Cryan. “Experiments like this are helping us move away from just describing changes and toward causative effects.”

The animal evidence seems promising, but translating this treatment to humans is still in the very early stages. OpenBiome, a nonprofit stool bank based in Cambridge, Massachusetts, is planning the first trials of fecal microbiota transplants for depressed patients. “We will try to select a donor that would complement the deficiency or replenish those bacterial communities that are depleted in patients with depression,” says Shrish Budree, senior clinical research scientist at OpenBiome (Figure 4, right). “One great aspect of OpenBiome is that we highly characterize all our stool donors,” Burdree notes. “They go through a rigorous clinical and serological screening, and we also genetically sequence all our donors so we know the exact microbial composition of their stool. If we know that certain bacterial communities are abnormal in a recipient, we can select a donor who is enriched in those bacteria.”
OpenBiome and its collaborators will be looking at the safety and efficacy of fecal microbiota transplants as well as the best mode of delivery (colonoscopy, nasogastric, or capsules). Another important aspect of the trial is to confirm whether the transplant alters the recipient’s microbiome profile, which researchers will accomplish by sequencing the recipient pre- and post-transplant and comparing that profile to the donor’s. Positive results in these initial trials could spur larger studies in the future.
Healthy Guts, Healthy Minds
Although the microbiome–gut–brain axis is now widely recognized, the specific mechanisms and microorganisms involved remain poorly understood. “In my view, the most plausible model is one in which there is a circular communication between brain, gut, and microbes,” says Mayer. “It probably starts with altered autonomic nervous system activity altering gut motility and secretion, affecting gut microbial composition and metabolite production. This, then, acts on the brain to affect networks involved in mood and motivation.”
A basic challenge is that scientists have not yet been able to comprehensively identify and characterize the composition of a healthy gut. There is a great deal of individual variability in gut microbiota and little consensus on what bacterial species are most involved in depression.
Preclinical research suggests that the gut microbiome could be a target for preventing or treating depression, but more work, especially in humans, is needed. Scientists are continuing to assess the efficacy of probiotics, diet, and fecal microbiota transplantation in animal models as well as working to confirm confirm what they find in human patients. “We might one day treat depression by treating the actual microbiome,” Cryan asserts. “Targeted, personalized microbiome-based diets, whose purpose would be to ramp up specific bacteria and the chemicals they produce, might not be that far off.”
One of the next steps is identifying those depressed patients who would benefit from microbiome-based treatments. “I think the microbiome–gut–brain axis is a great proxy for differences across individuals,” says Foster. She adds, “It might reduce some of the heterogeneity in mental health. We are working on using microbiome–gut–brain axis measures, such as blood levels of bacterial products, as biomarkers to subtype patients. If we can predict which people will respond to which types of treatment, we can get precision medicine—getting the right treatment to those who will benefit—moving faster.”
Over the next decade or so, physicians might be able to determine which bacteria are missing in depressed patients and supplement their gut with probiotics, diet recommendations, or fecal microbiota transplants. This approach likely won’t replace current treatments for depression, but it could prove to be another tool in the arsenal for some patients.
Additional Resources
- S. Dash, G. Clarke, M. Berk, and F. N. Jacka, “The gut microbiome and diet in psychiatry: Focus on depression,” Current Opinion Psychiatry, vol. 28, no. 1, pp. 1–6, 2015.
- A. Evrensel and M. E. Ceylan, “The gut-brain axis: The missing link in depression,” Clinical Psychopharmacology Neurosci., vol. 13, no. 3, pp. 239–244, 2015.
- J. A. Foster and K. M. Neufeld, “Gut-brain axis: How the microbiome influences anxiety and depression,” Trends Neurosci., vol. 36, no. 5, pp. 305–312, 2013.
- R. A. Luna and J. A. Foster, “Gut-brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression,” Current Opinion Biotechnology, vol. 32, pp. 35–41, Apr. 2015.
- G. B. Rogers, D. J. Keating, R. L. Young, M.-L. Wong, J. Licinio, and S. Wesselingh, “From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways,” Molecular Psychiatry, vol. 21, pp. 738–748, Apr. 2016.
- E. Sherwin, K. Rea, T. G. Dinan, and J. F. Cryan, “A gut (microbiome) feeling about the brain,” Current Opinion Gastroenterology, vol. 32, no. 2, pp. 96–102, 2016.