It’s a bacteriophage’s world; we’re just living in it
Bacteriophages, or phages for short, are viruses that specifically infect bacterial cells. Their name is derived from Greek for “bacteria eater” and it’s an apt description: To reproduce, they must coopt the cellular machinery of a bacterium, sometimes destroying it in the process [1]. Phages thrive anywhere bacteria exist—on land, in water, and inside plants and animals. In fact, they are considered the most successful organisms on earth due to their abundance and genetic diversity. Scientists estimate there are a trillion phages for every grain of sand on earth. That’s 1031 phages [2].
Thousands of varieties of phage exist, each evolved to infect only one or a select few types of bacteria. This lethal specificity is one reason that phages have been used to combat antibiotic-resistant infections [3]. “If you choose any bacterium that infects somebody, you’re almost certain to find a virus that can attack and kill it,” says Carl Merril, a National Institutes of Health Emeritus Scientist and co-founder and CSO of the company Adaptive Phage Therapeutics (APT) (Figure 1).

Figure 1. Carl Merril is a National Institutes of Health Emeritus Scientist and co-founder and CSO of the company APT. (Photo courtesy of APT.)
Phages are proven, powerful tools to treat multidrug antibiotic resistant infections. But their medical utility could go even further. A few research groups and biotech companies are exploring the potential of phages in vaccine development. And when the COVID-19 pandemic presented a challenging, new target, some researchers were ready to put phages into action.
Why phages?
Phages possess several characteristics that make them suitable for vaccine development. First, phages are relatively easy and inexpensive to produce in large quantities. They are also highly stable under harsh environmental conditions, meaning they would not require a complex cold chain for distribution and storage. In addition, phages have demonstrated an ability to activate both the innate and adaptive immune systems. Finally, because phages only infect bacterial cells, they are safe for use in humans and don’t cause any significant side effects [4].
The turning point in leveraging phages in vaccine development was the introduction of phage display technology in 1985. Phages have two components, their DNA and an outer protein coating or capsid. In phage display, a gene sequence encoding a peptide or protein of interest is inserted into a capsid gene of the phage. The peptide or protein is subsequently displayed on the surface of the phage [5]. For vaccine purposes, foreign antigens can be displayed on the surface of phages to stimulate an immune response.
According to Merril, phage display technology allows researchers to take phages apart and put them back together like Lego blocks. “We can put anything we want on their surface to stimulate our immune systems to react against,” he says.
Fighting infectious diseases
Venigalla Rao (Figure 2), a biologist at Catholic University, was introduced to phages as a post-doctoral researcher. “I became fascinated by phages and kind of fell in love with the phage known as T4,” he says. Now, more than 40 years later, Rao’s work on T4’s genetics and structure have resulted in significant advances in our understanding of its function and mechanisms, as well as enabling novel biomedical applications.

Figure 2. Venigalla Rao is a biologist at Catholic University. Rao’s work on the basic biology of T4 has allowed his team to explore biomedical applications of the phage. (Photo courtesy of Venigalla Rao.)
T4 is a phage that infects E. coli, the harmless bacteria that live in our guts. Rao’s research has laid the groundwork for developing the phage as a vehicle to deliver new vaccines and gene therapies into the human body. He and his team accomplish this by adding proteins to the outer surface of its capsid and packaging therapeutic DNA molecules in its interior. In addition to phage display technology, Rao’s lab uses the gene editing tool CRISPR to rapidly construct recombinant phages for vaccine and gene delivery [6].
Rao’s work on the basic biology of T4 has allowed his team to explore biomedical applications of the phage, including the design of a dual anthrax–plague vaccine that has shown success in protecting mice from the two deadly pathogens [7]. “We could take pieces of those pathogens that cause anthrax and plague and display them on the T4 capsid, ultimately producing a vaccine that can protect against both diseases,” he says.
In 2020, Rao’s team was working on another application for T4 phages: a universal flu virus vaccine. They were planning to use the flu as a model to design a universal vaccine template that could be adapted quickly to any emerging pathogen. But within a couple months, a new deadly pathogen would upset those plans and have Rao and other researchers pivoting their research focus.
Targeting COVID-19
At the beginning of the COVID-19 pandemic, many labs closed, except for those doing work directly related to SARS-CoV-2. Since Rao and his team had the T4 phage platform and the technology to incorporate any pathogen into a vaccine, they went to work on the new challenge. Their efforts resulted in a phage-based vaccine candidate capable of blocking SARS-CoV-2 infection in cultured cells and producing strong antibody titers in immunized mice [8].
The research garnered the attention of APT, expanding the biotech company’s focus from using phages to fight drug-resistant infections to also developing a phage-based COVID-19 vaccine. APT, with grant support from the U.S. Department of Defense, has partnered with Catholic University to develop Rao’s vaccine (Figure 3). If Phase 1 trials are successful, they plan to advance the T4 vaccine through Phase 3 clinical studies in humans [6].
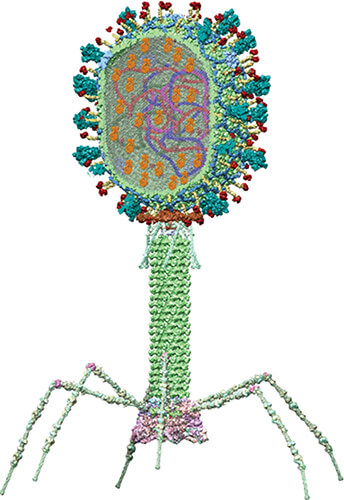
Figure 3. Structural model of Catholic University’s COVID vaccine showing the SARS-CoV-2 viral components incorporated into a T4 bacteriophage; genes (strings of blue, red, and purple), nucleocapsid proteins (dark brown globules inside capsid), and spike protein trimmers (cyan-colored spikes on the capsid surface). (Image courtesy of Victor Padilla Sanchez, Ph.D.)
One novel aspect of this vaccine is its mode of delivery: Rao and APT are exploring the potential for an oral delivery system in the form of a lozenge or soluble film. Rao says this would make distribution even easier and even enable the vaccine to be mailed to people.
Another potential benefit may be the phage-based vaccine’s adaptability to virus mutations. Although the currently available COVID-19 vaccines have been a powerful tool in combatting the virus, they are not as effective against some emerging variants. Rao says that whereas the current vaccines incorporate only one antigen, the coronavirus spike protein, his T4 vaccine can display more COVID-19 proteins, thus providing broader immunity against the virus and potentially against current and future variants.
“I think our COVID-19 vaccine may have more long-term efficacy, especially against the variants,” says Rao. “A combination of spike and nucleocapsid proteins may be more effective for the emerging variants than the spike-specific vaccines currently in use.”
Phages versus cancer
Another area where phages are proving useful is in cancer treatment. Standard cancer treatments such as chemotherapy drugs lack specificity and may result in toxicity and widespread damage of healthy cells in addition to cancer cells. Phages show promise as a means to target drug delivery directly to tumor cells.
In addition, phages are capable of modifying the tumor microenvironment, which is notoriously difficult to infiltrate. Phages tend to elicit strong responses from the innate immune system and can alert the body to the tumor, effectively “waking up” the immune system to fight the cancer [9].Renata Pasqualini (Figure 4), chief of the Division of Cancer Biology at Rutgers Cancer Institute of New Jersey and CSO of PhageNova Bio, knows the cancer-fighting power of phages well. “I started my career leveraging phages as viruses that could be bioengineered for both discovery and medical applications,” she says. For more than 25 years, Pasqualini, with husband and scientific collaborator Wadih Arap, has researched novel methods of combatting cancer. Phages are one of the tools they use. “We’ve engineered phages to deliver genes to tumors in order to either kill cells that are undesirable or image tumors in a very selective way,” says Pasqualini.

Figure 4. Renata Pasqualini is chief of the Division of Cancer Biology at the Rutgers Cancer Institute of New Jersey and CSO of PhageNova Bio. Pasqualini has been working to develop a phage therapy for cancer tumors. In addition, Pasqualini’s team, including researchers from Northeastern University, Rice University, and Rutgers University, recently published a proof-of-concept study detailing two phage-based approaches to vaccinating against the SARS-CoV-2 virus. (Photo courtesy of Renata Pasqualini.)
Expanding the reach of vaccines
Like Rao, Pasqualini’s team had the knowledge and platform to respond when the COVID-19 pandemic began in 2020. “Instead of shutting down, we shifted our focus to leverage the same systems we were already using in oncology to create phages that could be used to immunize against SARS-CoV-2,” she says.
Pasqualini’s team, including researchers from Northeastern University, Rice University, and Rutgers University, recently published a proof-of-concept study detailing two phage-based approaches to vaccinating against the SARS-CoV-2 virus, both of which produced strong systemic antibody responses in mice [10]. The first approach, which can be delivered via aerosol inhalation, uses phage particles engineered to display the SARS-CoV-2 spike protein along with a small ligand peptide that helps the phage cross from the lungs to the bloodstream. The second approach uses injectable engineered phage particles to deliver the gene encoding the spike protein.
In 2020, Pasqualini’s company, PhageNova Bio, entered into an exclusive licensing agreement with Rutgers to develop these vaccine constructs. The project is still in its early stages, but the team sees a role for their phage-based vaccine in expanding the reach of COVID-19 vaccines. Both of the approaches developed by Pasqualini and colleagues are scalable, straightforward to genetically engineer or modify, and can be transported and stored at room temperature, making them logistically simple to disperse and deliver to rural and remote communities around the world.
“Our vaccine would be stable, wouldn’t require a cold chain for distribution, and could be affordable and accessible to anybody, including underserved populations in poor countries,” says Pasqualini. “If we really want to do away with COVID-19 or any other pandemic that may arise down the road, we will have to vaccinate the entire planet. Phage-based vaccines could make a difference since they can be manufactured quickly and easily and they are stable for long periods of time.”
Phage-based vaccines may be able to fill unmet public health needs, but it will take more development, according to Pasqualini. “There have been proof-of-concept studies, but they have not reached the point at which they have been tested in primate models or clinical trials,” she says. “That will require more of an investment in the biotech space. It hasn’t happened yet, but that doesn’t mean it won’t happen.”
References
- UC San Diego Health. Phage 101. Feb. 21, 2022. [Online]. Available: https://health.ucsd.edu/news/topics/phage-therapy/Pages/Phage-101.aspx
- L. Aghebati-Maleki et al., “Phage display as a promising approach for vaccine development,” J. Biomed. Sci., vol. 23, no. 1, p. 66, Dec. 2016, doi: 10.1186/s12929-016-0285-9.
- L. Mertz, “Battling superbugs: How phage therapy went from obscure to promising,” IEEE Pulse, vol. 10, no. 1, pp. 3–9, Jan./Feb. 2019. [Online]. Available: https://www.embs.org/pulse/articles/battling-superbugs-how-phage-therapy-went-from-obscure-to-promising/
- A. González-Mora et al., “Bacteriophage-based vaccines: A potent approach for antigen delivery,” Vaccines, vol. 8, no. 3, p. 504, Sep. 2020, doi: 10.3390/vaccines8030504.
- K. L. Hess and C. M. Jewell, “Phage display as a tool for vaccine and immunotherapy development,” Bioeng. Transl. Med., vol. 5, no. 1, p. e10142, Jan. 2020, doi: 10.1002/btm2.10142.
- E. N. Woods,“Viral sensation,” Catholic Uni. Mag., Fall 2021. [Online]. Available: https://communications.catholic.edu/magazine/fall-2021/viral-sensation.html
- P. Tao et al., “A bacteriophage T4 nanoparticle-based dual vaccine against anthrax and plague,” mBio, vol. 9, no. 5, p. e01926, Nov. 2018, doi: 10.1128/mBio.01926-18.
- J. Zhu et al., “A universal bacteriophage T4 nanoparticle platform to design multiplex SARS-CoV-2 vaccine candidates by CRISPR engineering,” Sci. Adv., vol. 7, no. 37, Sep. 2021, Art. no. eabh1547, doi: 10.1126/sciadv.abh1547.
- V. Foglizzo and S. Marchiò, “Bacteriophages as therapeutic and diagnostic vehicles in cancer,” Pharmaceuticals, vol. 14, no. 2, p. 161, Feb. 2021, doi: 10.3390/ph14020161.
- D. I. Staquicini et al., “Design and proof of concept for targeted phage-based COVID-19 vaccination strategies with a streamlined cold-free supply chain,” Proc. Nat. Acad. Sci. USA, vol. 118, no. 30, Jul. 2021, Art. no. e2105739118, doi: 10.1073/pnas.2105739118.