When Northwestern University near Chicago, Illinois, announced in August 2015 that it had hired away “soft electronics” pioneer John Rogers from the University of Illinois at Urbana-Champaign, the exuberant reports in Chicago and the agonized ones downstate all shared similar descriptions of the man—who was also being wooed by a major California university at the time: “an emerging scientific star,” “a genius,” “a rockstar.” As University of Illinois chemistry professor Ralph Nuzzo put it in the campus newspaper The Daily Illini, “John is to engineering science what Mozart is to music. He’s just one of those rare kind of people, a fantastic, first-tier talent.”
And Rogers can indeed boast a long list of accolades. In 2009, at the age of 42, he won a MacArthur Genius Grant. In 2011 came the Lemelson-Massachusetts Institute of Technology (MIT) Prize for invention. He has received more than 80 patents. In September 2015, he took charge of a new US$25 million research facility Northwestern built for him, the Center for Bio-Integrated Electronics. Benefactors to the university donated that money specifically to lure him there.
The name of the center perfectly captures the vision that’s been driving Rogers’ research for well over a decade, leading him to spawn ten companies. A unifying theme of Rogers’ work: make electronic devices less like hard, sharp-edged, brittle objects and more like the human body—rounded, soft, and flexible. The ultimate goal of all the softness: easier interaction with biological tissue for diagnostic and treatment applications.
Rogers’ quest for soft and squishy electronics took off with his publication of a paper in Science in 2011, in which he introduced the idea of “epidermal electronics,” functioning circuitry—albeit fairly basic at that time—nearly as thin and stretchy as skin. That first proof-of-concept “opened up whole new possibilities for integrating skin-mounted devices” for all kinds of health-monitoring purposes, he says.
Now, the first commercial fruits of that vision are about to arrive. One of them is a flexible patch, a device as small and thin as a postage stamp, that could soon be providing health-monitoring capabilities that match and, in some cases, potentially improve on tests that now require a visit to a hospital or clinic.
The patch is as thin as a temporary tattoo, so thin that the person wearing it can barely feel it. Air and moisture pass right through it, and it flexes when one moves, just as the skin itself does. But embedded within it is a set of sensors that can continuously monitor a person’s movements, heartbeat, temperature, and a variety of other parameters, sending them to a smartphone or tablet via Bluetooth, and from there to the cloud, where a doctor can check a detailed record of that person’s physical activity and vital signs over a stretch of days or even weeks.
Such ongoing monitoring makes it possible, for example, to pick up subtle, intermittent signs of a progressive movement disorder such as Parkinson’s disease, or variations in temperature that could provide early warning of a building infection, or occasional irregularities in heartbeat that might be precursors of a heart attack but never show up during the limited time span of tests done in a clinic or a doctor’s office.
“I’ve worn it as long as four weeks,” explains Rogers, who is a cofounder of a company called MC10 that is refining and preparing to commercialize these skin-wearable sensors. And his personal wearing and testing of the device—and pushing it to its limits—is typical of the hands-on drive Rogers brings to his quest for better physical interfaces between humans and technology. The first versions of the patch, which he calls a “Biostamp,” were beta-tested by the U.S. Air Force last September. The product is scheduled to officially launch early in 2016.
The length of time people will be able to wear the device is not limited by the durability of the device, he explains, but by the properties of the skin itself: “The duration is not so much limited by adhesion or discomfort; it’s really a consequence of the natural biological process, the dead cells that are constantly exfoliating.” Once the product is on the market, he says, it will likely be recommended for use for up to two weeks, “but during that two weeks you can do anything, take showers, even go swimming.” And once it has done its work of collecting data, the patch can be removed as easily as an adhesive bandage, rinsed off, and be ready to use again.
Rogers got his doctorate degree in physical chemistry from MIT. During his studies, he met his wife, materials scientist Lisa Dhar. Soon after Rogers earned his degree, the couple entered a startup competition that led to the founding of his first company. That company was based on technology developed as part of his thesis work on monitoring materials distantly using lasers. From there, he spent time at Bell Labs directing its condensed-matter physics lab, before ending up in 2013 at the University of Illinois, where he took up his quest for a more biologically compatible way to build devices.
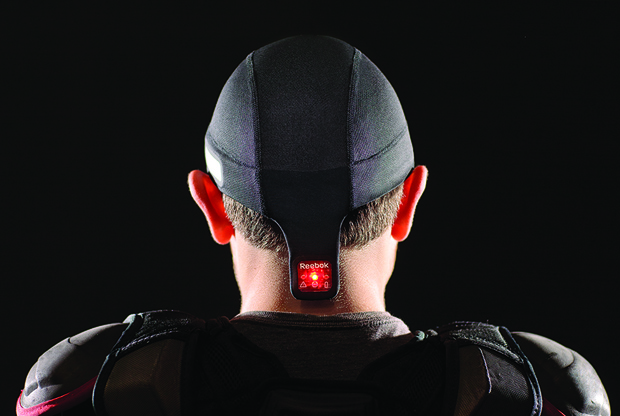
Started in 2008, MC10 made its debut in the marketplace in the spring of 2015 with a product called “Checklight,” designed to help identify athletic head injuries (Figure 1). Distributed through a partnership with Reebok, the product is a thin, flexible skullcap embedded with gyros and accelerometers and designed to be worn under a sports helmet. In the event of a blow to the head during the course of a game, the device can provide an immediate readout of the force of the impact, giving coaches and parents some quantitative data to help them make a more accurate assessment of whether a concussion has occurred and the player should be taken out of the game.
But MC10 has also been carrying out extensive tests on a variety of the ultrathin skin-mounted Biostamps that are already in use for research. In the coming years, it intends to have consumer versions available for both fitness tracking and health monitoring. The devices are intended to be used in the home, at the gym, in clinics and hospitals, and just about anywhere else.
Among the first devices available for clinical research purposes will be highly sensitive motion trackers, notes Roozbeh Ghaffari (Figure 2), MC10’s vice-president of technology and a company cofounder along with Rogers and Carmichael Roberts, who serves as chair. While a host of devices for motion tracking are on the market today, mostly for fitness monitoring, these are simple, wrist-mounted monitors that give only a very basic kind of information, such as number of steps or distance walked. The small, lightweight, and flexible nature of MC10’s patches makes it possible to wear several of them at once on different parts of the body, allowing a very detailed picture of a person’s total physical activity, at high resolution.

Rogers says the MC10 system will be able to provide data on “full-body kinetics.” As he describes it, “You can put them anywhere on the body, not just the wrist. We’ve done clinical tests with up to seven on different parts of the body, and we can do storage of data from all of these simultaneously. We get really accurate data on range of motion of your body, so it’s useful for characterizing motion disorders.”
All this comes from a package so vanishingly thin that, as Rogers explains, “you can’t handle them directly; you peel off a backing to put them on and then peel the top layer off.” The material “is designed to match the stiffness of the skin itself,” he notes, and “to go on in a way that doesn’t constrain any of the normal properties of the skin. It’s gas and vapor permeable, so you don’t trap water.”
There are three key areas of innovation that make the thin, flexible electronics package possible, Ghaffari says—and all of them flowed from Rogers’ research and his dogged pursuit of flexibility.
“First is the ability to build ultrathin, bare-die electronics,” he suggests. These use conventional microchip manufacturing methods but with a layered system, which took years of painstaking development in Rogers’ lab, that allows the topmost thin layer of the silicon wafer, as little as a few hundred nanometers thick, to be peeled off from the bulk of the wafer.
Second, the company has a novel way of interconnecting these ultrathin components, using a system Rogers devised. “It’s a springlike interconnect, which provides active routing lines for allowing signals from the processor to each of the sensors,” according to Ghaffari. The brittle interconnects used in conventional circuitry tend to break when flexed, but these serpentine wires instead “can bend and stretch.”
And, finally, the pieces have to be put together in a wearable form, using a thin and flexible skin-compatible material—an area where Rogers’ work has been “on the cutting edge,” Ghaffari says. “We did a lot of study on biocompatible materials and very breathable interfaces.” A key aspect of the design was creating an effective interface with the skin below the device.
That tight fit is not just a matter of comfort. It’s that intimate bond with the skin that gives the system the potential to go far beyond existing devices worn on wristbands or thicker kinds of stick-on patches, as Rogers elaborates. “It’s critically important to make the device comfortable and wearable and nonirritating. But it’s also important in capturing high-quality data. It eliminates lots of the sources of motion artifacts that affect most wearable devices.” For many of the kinds of measurements the devices can make, he says, “It yields data that compare very favorably with hospital gold standards.”
One example of such high-resolution data is, perhaps surprisingly, for temperature. Even Rogers was at first skeptical of the usefulness of such measurements.
The U.S. National Institutes of Health (NIH) had a research project on “precision thermography,” he explains, “and they reached out to us, but it didn’t seem to us that exciting. You have a temperature or you don’t. But it turns out if you can measure temperature to the third decimal point, you can pull out very delicate effects.”
And with the tight contact offered by the Biostamp, it turns out MC10 could indeed achieve the millikelvin level of accuracy the NIH was looking for.
“The pattern of blood flow in the direct area of the skin affects the skin temperature at the surface. They studied it with very high-end infrared cameras, each of which cost a quarter-million dollars, but you can see all kinds of subtle effects, minima and maxima and small gradations,” Rogers says. “Well, it’s comparable to the kind of precision we can get—we can match that precision, and, for us, the materials cost is something like five cents.”
In fact, the skin patch has some real advantages beyond just cost. “The most important thing is to eliminate motion artifacts,” the fluctuations in the readings caused by movements of the body but that look like those caused by physiological changes. “The cameras only work if the patient is strapped down. But the patch just moves along. And we can use one temperature sensor or as many as you want.”
That’s just one of a host of parameters that MC10 sensors now undergoing testing or in the development pipeline can detect. According to Rogers, the company is currently working on devices to measure blood-flow dynamics, flow rates, and pressure wave velocity, which can be converted to a measure of blood pressure variations, and they also use light-emitting diodes and photodetectors to detect pulse oximetry, the level of oxygenation in the blood. By injecting tiny pulses of heat, the devices can measure the skin’s thermal conductivity, which is related to hydration level. They can measure skin electrical conductivity, “which is directly related to the stiffness of the skin and can be a first-line measure of development of skin cancer,” Rogers says. They can also produce electroencephalograph and electrocardiogram readings.
Even though these products are all in the testing phase and not yet on the market, Rogers is already looking ahead to a next generation of devices. For example, instead of taking measurements only from the surface of the skin, more detailed biomedical data are available if the devices can penetrate even a minuscule depth into the skin itself. He and the MC10 team are hard at work on the necessary technologies. “We’ve been able to extract fluid, interstitial fluid, and even blood, using a fluidic interface on the skin,” he explains. “Then you can do all kinds of chemical analysis.” But there’s a lot of work still to do in that arena. “We know how to do soft fluidics now, and we have devices that go on the skin and capture sweat into reservoirs to do basic chemical analysis. What we don’t know now is how to trigger microneedles and how to get fluid down through the skin.”
Still, considering how far the team has already come in developing devices that do a dizzying array of tests in a package that’s barely there, those further developments are likely to be not far behind. It is the chemical signaling that takes place in those fluids that provides some of the most important signaling pathways in the living body. “We need to incorporate a capacity for handling fluids,” Rogers says. “That will allow us to come in touch with the main language of biology”—the language of biochemistry.
And it is that kind of direct contact with biology that, in large part, motivated Rogers’ move to Northwestern: its close ties to a medical school and hospital that will facilitate working on clinical trials as he works to bring his ideas into real-world use. In fact, he will hold a joint appointment at Northwestern, in both the McCormick School of Engineering and the Feinberg School of Medicine.
“Many of our technologies are ready to move out of the lab and into clinical settings now,” Rogers told Crain’s Chicago Business about the impending move. Being in such close contact with a medical school, he said, “will be very helpful in that process.”



