Our bodies have the amazing ability to self-trigger tissue regeneration when required to repair or renew tissues. Adult stem cells emerge from their niche and are instructed by local environmental factors to differentiate into the needed specific phenotypes once they have migrated to the target sites. For example, the complex weaving of collagen filaments composing the tissue framework not only offers nanoscaled topographical cues to cells but also its stiffness may influence their differentiation. Specific biomolecules, secreted by glands or other cells and transported by body fluids, also provide instructive indications to cells. In this sense, combinations of various physical and chemical stimuli at the micro- and nanoscale are crucial in regenerating tissues.
However, seriously debilitated tissues, including those found after infarction, spinal injury, or large skin burns, may lose their capacity to provide cells with proper or sufficient instructive stimuli. In such situations, external intervention is required to support cellular activity with organ transplantation or grafting with healthy harvested tissues or biomaterials. Based on the instructive characteristics of the body, an emerging design strategy for biomaterials is to no longer attempt to recreate the tissue complexity in vitro but rather to focus on the development of biomaterials that have specific local characteristics, including surface stiffness, chemistry, and topography. These biomaterials are then able to actively engage with stem cells in vivo and direct them to have specific behaviors, ultimately leading to new tissue synthesis. Therefore, biomimicry strategies have been pursued to develop biomaterials that could mimic the specific tissue matrix characteristics.
Mimicking Tissue Characteristics
Some tissues—bone, for example—have a matrix composed by two or more chemically and physically different phases that determine their biological and mechanical properties. Inspired by this, various biomaterials have now been developed to mimic the multiphasic characteristic of tissues. In this scenario, the newly developed biomaterials may offer, in addition to instructive cues, mechanical characteristics close to those of the chosen tissue. In the case of bone tissue regeneration, biomaterials can mimic it structurally or biochemically by using either inorganics or their combinations with organics. When (porous) polymer materials are considered, bone may be structurally mimicked, but the biomaterial is generally bioinert.
However, polymers can be rendered bioactive, i.e., capable of initiating chemical bonding to bone, when combined with calcium phosphates that biochemically mimic the inorganic phase of bone. Most interestingly, the surface architecture and stiffness could instruct cells to have an osteogenic response regardless of the chemistry. The importance of surface architectures is clearly shown when two tricalcium phosphate ceramics having either submicro-/nanoscaled (TCPS) or microscaled (TCPB) surface architecture were used alone for bone tissue regeneration. When implanted in the muscles of dogs and sheep, only TCPS triggered heterotopic bone formation (Figure 1).
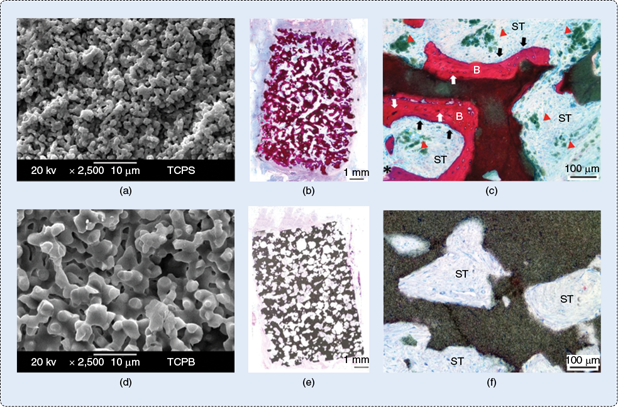
This suggests that manipulating the surface architecture at the submicro- or nanoscale level resulted in a unique subgroup of instructive biomaterials that have the potential to spur undifferentiated stem cells to differentiate into bone phenotype without addition of (osteogenic) factors or cells. Such osteoinductive materials have also been demonstrated to be effective in repairing critical-sized bone defects [1], [2]. Despite the clear biological benefits such osteoinductive calcium phosphate ceramics could bring, their brittleness is a major drawback. Therefore, designing nanoscaled osteoinductive composite biomaterials with improved mechanical properties is suggested as a possible way to overcome these issues.
Bone surface chemistry and roughness have been mimicked at the micro- or submicro-/nanoscale by letting calcium phosphates precipitate from ultrasaturated solutions (e.g., concentrated simulated body fluids) onto the surface of polymer-, peptides sheets-, or graphene-based bulks [3], [4]. The natural intrafibrillar collagen mineralization that occurs during bone tissue development has also been replicated in vitro to produce composite materials that chemically and hierarchically mimic the bone tissue [5]. Meanwhile, the macrostructure of bone could be imitated by using composites of silk fibers and calcium phosphate in the form of macroporous blocks with a mechanical strength similar to bone [6].
Nanoscaled calcium phosphates may be homogeneously introduced into polymers to obtain biphasic composites where the calcium phosphate phase would contribute to the biological properties while the polymer phase would determine the mechanical properties (Figure 2) [7], [8]. The presence and exposure of nanoscaled calcium phosphates in a polymeric matrix may generate nanoroughness that could enhance osteogenic differentiation of stem cells. However, the polymer may have effects or even actively take part in the material-directed biological responses, and, therefore, its role needs to be carefully considered as well. The challenge is developing osteoinductive composite materials that are, at the same time, degradable to leave room for the growing tissue, mechanically suitable for the required clinical application in load-bearing sites, and still be able to induce and/or guide bone formation.
![FIGURE 2 (a) A transmission electron microscopy (TEM) image of nanosized calcium phosphate apatite particles that can be used to produce nanocomposites that biochemically mimic bone. The scale bar is 50 nm. (b) A TEM image showing homogeneous distribution of nanosized apatite particles in a polylactide matrix [8]. The uniformity of apatite distribution is a crucial factor to obtain mechanically good materials. (c) An SEM image of the surface of a nanocomposite of polylactide and nanosized apatite particles. The nanostructured surface due to the exposure of apatite particles is shown. (d) An atomic force microscopy three-dimensional (AFM 3-D) image showing that the exposure of apatite particles gives to the surface of the composite a nanorough topography, which may later tune stem cells’ fate [9] (unpublished). (Used with permission from [8].)](https://www.embs.org/wp-content/uploads/2014/03/32871.png)
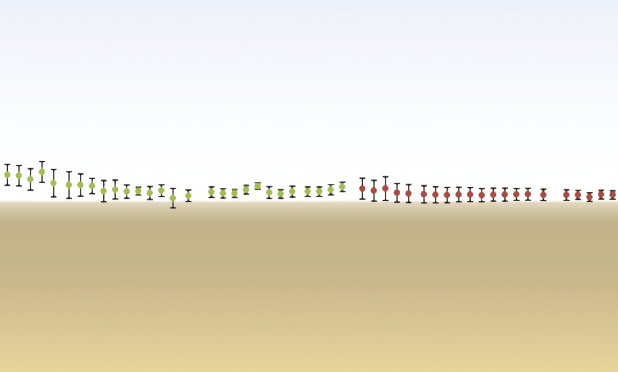
Designing such an instructive biomaterial with proper dynamic mechanical properties, degradation rate, and osteoinductive potential is challenging because various factors are involved that influence each other. Adding (submicro- or nano-) (hydroxy) apatite particulate or fibers into polylactide has led to materials with improved mechanical properties. In particular, composites with high hydroxyapatite content also have become bioactive with enhanced protein adsorption and osteoblast adhesion in vitro. However, the dynamical mechanical performances and damping properties have to be tuned until reaching those of dry bone by adjusting the apatite content. In wet conditions, such materials had inferior mechanical behavior than bone, highlighting the challenge to reach ideal mechanical properties [8]. Increasing the molecular weight of the polymer phase was suggested as well, but the biological characteristics of the composite worsened and vice versa (Figure 3) [7].
![FIGURE 3 After a six-month intramuscular implantation in a sheep, heterotopic bone formation was triggered by (a), (b) the composite material that contained lower molecular weight polymer but not by (c), (d) the one with the same polymer but higher molecular weight. Immature woven and mineralized bone tissue is indicated by white stars, which is covered on its outer by a seam of active osteoblasts (black arrows). Either surface mineralization or layers of apatite particles exposed by the degradation are visible on the granule surface (white arrows) in both materials. ST: soft tissue; BM: bone marrow; V: blood vessels. (e), (f) Three-point bending dynamic mechanical measurements in dry air revealed that the composites had comparable (e) stiffness and (f) damping characteristics to those of human dry cortical bone [7]. The red line refers to the mechanical properties of composite that could induce bone formation, while the green one refers to the material that contains higher molecular weight polymer but was not able to trigger bone formation. (Figure adapted and reprinted with permission from [7].)](https://www.embs.org/wp-content/uploads/2014/03/32911.png)
These examples show that simply varying one material’s factor could be detrimental for the performance efficacy of the biomaterial. Indeed, a balance between sufficient mechanical characteristics and biological properties could be reached by focusing on a few mechanisms occurring in nature that effectively influence and control cell fate and trying to reproduce them in a material. In particular, in view of a simple and effective approach, research should concentrate on simple and controllable intrinsic properties of biomaterials able to interact with the biological environment by means of protein adsorption, surface mineralization, and the release of certain ions that will influence cell behavior.
One tactic has been to tune the molecular weight of the polymer phase to enhance body fluid uptake. As body fluids carry various molecules and ions, it is possible that a larger uptake of fluids would improve the contact between such biomolecules and the material’s surface, enhancing their adsorption and the surface mineralization. This may indirectly enhance the early cell response upon implantation, triggering macrophages to secrete cytokines that could later induce bone induction. Furthermore, absorbed fluids would accelerate the polymer hydrolysis and calcium phosphate dissolution, enhancing the degradation of biomaterials and facilitating the release of calcium and phosphate ions. Degradation of biomaterials may also generate changes at the surface, e.g., by exposing more calcium phosphates or generating nano- or microporosity. The combination of such phenomena may be triggered by the fluid uptake, which was in turn tuned by the molecular weight of the polymer phase. These processes eventually contributed to the heterotopic bone formation in nanoscaled composite materials [7].
Moving Beyond Bone
A similar approach of inducing specific surface phenomena by controlling biomaterials’ properties has also been shown in the regeneration of tissues other than bone, e.g., vascular tissue. In one study, nanostructured titanium with enhanced surface nanoroughness positively tuned the synthesis of elastin and collagen by endothelial cells, indicating improved adhesion [10]. Other studies have shown that a combination of alkali etching and cast molding of polymeric materials led to random nanometer structures that, besides enhancing fibronectin and vitronectin adsorption from serum, also promoted endothelial and vascular smooth muscle cell proliferation. In addition, polymer surfaces with nanospherical patterns enhanced fibronectin adsorption, leading to enhanced endothelial and smooth muscular cell responses as compared to smoother or rougher counterparts. Thus, cells have been shown to respond in different manners to various combinations of geometrical, physical, and chemical characteristics of biomaterials’ surface, indicating that nanomaterials may have a huge potential to specifically instruct cells toward particular biological responses.
To mimic the fibrous tissue networks, matrices of polymer micro- or nanofibers have been produced by electrospinning and proposed for many applications in regenerative medicine, including the repair of dura mater, nerves, tendons, ligaments, and blood vessels. Exciting results have been reported with electrospun poly(l-lactide) nanofibers incorporating laminin, which structurally and biochemically mimicked the neural tissue environment, and showed the ability to induce peripheral nerve formation by cells derived from rat adrenal medulla. Besides nanotopographical and biochemical cues from the extracellular matrix, neural tissue also contains electrical signals. Therefore, nanomaterials with electrical properties have been proposed. Examples are carbon nanotubes and fibers, which have strong mechanical properties and are electrical conductors. Because of their electrical conductivity, they have been studied as biomimetic guides for axon regeneration and to repair injured neural tissue.
In other studies, neurons have been observed growing and developing well-branched dendrites on positively charged multiwalled carbon nanotubes, and neuronal differentiations of rat neuronal medulla cells and branches development were favored onto vertically aligned carbon nanotubes arrays presenting electrical conductivity [11], [12]. It was postulated that such a surface mimicked, both structurally and electrically, the neural tissue matrix and guaranteed a suitable cell–surface interface for neural tissue regeneration purposes.
The role that nanotopography plays in influencing neural cells’ fate was further shown when rat cortical tissue cells were seeded onto smooth substrates presenting a pattern of carbon nanotube islands, and the cells preferentially attached to the islands and developed neural networks [13], [14]. Mimicry of the hydrated 3-D structure of natural (soft) tissue matrices (e.g., cartilage) was suggested by using hydrogels containing collagen peptide in combination with human mesenchymal stem cells, which interacted at the nanoscale level eventually leading to chondrogenesis. The urinary bladder has also an oriented nanostructured tissue framework, which has been mimicked with electrospun polymer fibers that guided the alignment of smooth muscle cells and enhanced their functions. In experiments, it was shown that the degree of surface nanoroughness was crucial in tuning the cells’ performances. Nanostructured synthetic polymers supported the adhesion and proliferation of human bladder smooth muscle cells that later synthesized matrices, ultimately leading to the formation of synthetic bladders.
However, although hydrogels and electrospun fibrous matrices may elegantly mimic the physicochemical and topographical features of the body tissue matrices at the nanometer scale, they do not present or guarantee similar mechanical performances as those in natural tissues. To overcome this problem, rapid prototyping techniques have been proposed, but they still cannot provide frameworks with nanodimensional features, which is the level at which most biological phenomena occur. Immobilizing bioactive molecules, such as those present in tissues, onto the surface of biomaterials has been suggested to mimic the instructive characteristics given by signaling molecules dispersed in the natural environments. Even so, it remains challenging to design a biomaterial that has multiple controllable characteristics.
In addition to the human benefits, instructive biomaterials research may also financially benefit the holders of clinically successful tissue engineering concepts. However, a combination of clinical performance, simplicity in use, cost effectiveness, and marketing is necessary for a successful business. Therefore, the instructive biomaterials must provide complex information, through their physicochemical properties, to direct cells toward tissue regeneration. Interest is growing in the exciting possibility of using simple material properties that physically or chemically influence cell behavior. Currently, researchers are actively pursuing simple but effective solutions to tissue engineering problems such that the ideal of structurally simple, yet functionally complex, biomaterials will be realized in the near future. For example, osteoinductive microstructured calcium phosphate ceramics have already widely shown their potential in instructing cells by means of the simple control of their surface/chemistry and they would soon be accepted in the market as bone void fillers. At the same time, it has been shown that producing degradable composite materials that are able to instruct cells in vivo, with improved mechanical properties, is possible. Such composites can be obtained for load-bearing bone replacement by designing them with few proper intrinsic characteristics, such as choosing the polymer phase molecular weight and monomer or the inorganic filler content.
In closing, a balance between biomaterial design complexity and feasibility (from research, industrial, and financial points of view) is necessary but, thanks to the fast progress in science, exciting scenarios in developing nanomaterials that can actively interact with biological elements loom on the horizon.
References
- H. Yuan, H. Fernandes, P. Habibovic, J. de Boer, A. M. C. Barradas, A. de Ruiter, W. R. Walsh, C. A. van Blitterswijk, and J. D. de Bruijn, “Osteoinductive ceramics as a synthetic alternative to autologous bone grafting,” Proc. Natl. Acad. Sci., vol. 107, no. 31, p. 13614, 2010.
- P. Habibovic, H. Yuan, M. van den Doel, T. M. Sees, C. A. van Blitterswijk, and K. de Groot, “Relevance of osteoinductive biomaterials in critical-sized orthotopic defect,” J. Orthop. Res., vol. 24, no. 5, p. 867, 2006.
- S. Kim, S. H. Ku, S. Y. Lim, J. H. Kim, and C. B. Park, “Graphene-biomineral hybrid materials,” Adv. Mater., vol. 23, no. 17, p. 2009, 2011.
- S. Segman-Magidovich, H. Grisaru, T. Gitli, Y. Levi-Kalisman, and H. Rapaport, “Matrices of acidic b-sheet peptides as templates for calcium phosphate mineralization,” Adv. Mater., vol. 20, no. 11, p. 2156, 2008.
- Y. Liu, N. Li, Y. P. Qi, L. Dai, T. E. Bryan, J. Mao, D. H. Pashley, and F. R. Tay, “Intrafibrillar collagen mineralization produced by biomimetic hierarchical nanoapatite assembly,” Adv. Mater., vol. 23, no. 8, p. 975, 2011.
- A. M. Collins, N. J. V. Skaer, T. Gheysens, D. Knight, C. Betram, H. I. Roach, R. O. C. Oreffo, S. von Aulock, T. Baris, J. Skinner, and S. Mann, “Bone-like resorbable silk-based scaffold for load-bearing osteoregenerative applications,” Adv. Mater., vol. 21, no. 1, p. 75, 2009.
- D. Barbieri, H. Yuan, X. Luo, S. Farè, D. W. Grijpma, and J. D. de Bruijn, “Influence of polymer molecular weight in osteoinductive composites for bone tissue regeneration,” Acta Biomater., vol. 9, no. 12, p. 9401, 2013.
- D. Barbieri, J. D. de Bruijn, X. Luo, S. Farè, D. W. Grijpma, and H. Yuan, “Controlling dynamic mechanical properties and degradation of composites for bone regeneration by means of filler content,” J. Mech. Behav. Biomed. Mater., vol. 20, p. 162, Apr. 2013.
- D. Barbieri, J. D. de Bruijn, and H. Yuan, “Surface structure of nanocomposites and its properties: A practical example,” in Frontiers in Nanobiomedical Research: Tissue Regeneration: Where Nano Structure Meets Biology, Q. Liu and H. Wang, Eds. Singapore: World Scientific, 2013.
- S. Choudhary, K.M. Haberstroh, and T. J. Webster, “Enhanced functions of vascular cells on nanostructured Ti for improved stent applications,” Tissue Eng., Part A, vol. 13, no. 7, p. 1421, 2007.
- H. Hu, Y. Ni, V. Montana, R. C. Haddon, and V. Parpura, “Chemically functionalized carbon nanotubes as substrates for neuronal growth,” Nano Lett., vol. 4, no. 3, p. 507, 2004.
- T. D. B. Nguyen-Vu, H. Chen, A. M. Cassell, R. J. Andrews, M. Meyyappan, and J. Li, “Vertically aligned carbon nanofiber architecture as a multifunctional 3-D neural electrical interface,” IEEE Trans. Biomed. Eng., vol. 54, no. 6, p. 1121, 2007.
- T. Gabay, E. Jakobs, E. Ben-Jacob, and Y. Hanein, “Engineered self-organization of neural networks using carbon nanotube clusters,” Physica A, vol. 350, no. 3–4, p. 611, 2005.
- S. Q. Liu, Q. Tian, J. L. Hedrick, J. H. Po-Hui, P. L. Ee, and Y. Y. Yang, “Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage,” Biomaterials, vol. 31, no. 28, p. 7298, 2010.