A person who has had a myocardial infarction often gets excellent emergency treatment at a hospital, and is able to go home or even return to work in a matter of days. Despite this quick recovery, all is not perfect, because the infarction stops blood and oxygen flow, which can quickly kill heart tissue. Unlike many other types of human tissue, heart tissue doesn’t regenerate, so heart function is compromised.
New research projects are under way to change that. These include developing models both to peer more closely at cardiac infarction and to test treatment options, notably drugs, in a patient-specific manner; growing replacement heart tissue in the lab; and nudging the body to start producing its own replacement cardiac tissue. All three areas are moving ahead rapidly and showcase exciting opportunities for the future of cardiac care.
Chipping In
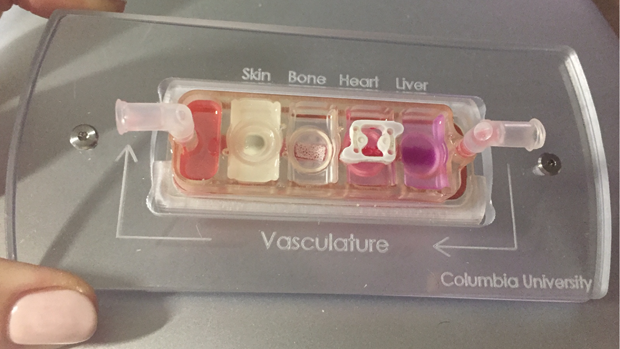
Significant research over the past two decades has gone toward the development of biomimetic models of cardiac tissue, called organ-on-a-chip or heart-on-a-chip (Figure 1). Research groups began making engineered-heart-tissue (EHT) models by using embryonic stem cells, differentiating them into heart cells, and using the cells to construct small pieces of 3D heart tissue. The end goal is to produce thick and vascularized tissue that is electro-mechanically functional, according to Ali Khademhosseini, professor of bioengineering and director of the Center for Minimally Invasive Therapeutics at the University of California, Los Angeles (UCLA) (Figure 2, below).

Ali Khademhosseini
Now about two decades into EHT development, research groups have expanded and refined techniques to make heart-tissue models. Khademhosseini’s group, for instance, is employing bioreactors to turn immature and undifferentiated stem cells into fully mature and functional heart cells. “We use bioreactors to provide mechanical or electrical pacing or stimulation so that the cells get exercise that matures all their architecture and contacts with each other [1],” he said. “We also add the right kinds of surrounding molecules, such as soluble proteins or matrix materials, around the cells so that they are in the right environment to help them to further mature.”
An additional challenge is keeping EHT alive. “Because these are very active cells and consume a lot of oxygen, vascularization and delivery of oxygen and nutrients becomes very important,” Khademhosseini said. For this task, he and his group turned to 3D printing [2] to build particular shapes and structures, and to add microfluidic networks for the delivery of blood throughout the three-dimensional tissue “so that we can make structures that look and act like heart tissue,” he described.
One of the other groups developing vascular-perfused, organ-on-a-chip technology to model human physiology is that of Gordana Vunjak-Novakovic, university professor and the Mikati Foundation Professor of Biomedical Engineering and Medicine at Columbia University (Figure 3). She and her team are especially interested in using the approach to look at individual patients and understand “how to use existing drugs more effectively, and how the progression of disease and recovery depends on patient-specific factors,” she said.

Her group has developed models by growing human heart muscle from induced pluripotent stem cells or induced pluripotent stem cells (iPSCs) [3], [4] and designed a heart-failure-on-a-chip model [5] to get a better grasp of the progression of injury or disease, as well as healing (Figure 4). “Our model mimics events during infarction, including the blocking of the blood flow that happens during myocardial infarction, and the re-establishment of blood flow, which is called reperfusion,” Vunjak-Novakovic explained. “This whole infarction–reperfusion process is very complex and is still not very well understood, so there are some really big advantages of modeling this in vitro using human tissue.” She added, “There have been real advances in the field as a result of heart-on-a-chip models, but every success opens five more questions, so it’s a very exciting time for us bioengineers.”
The Big Fix

Sian Harding
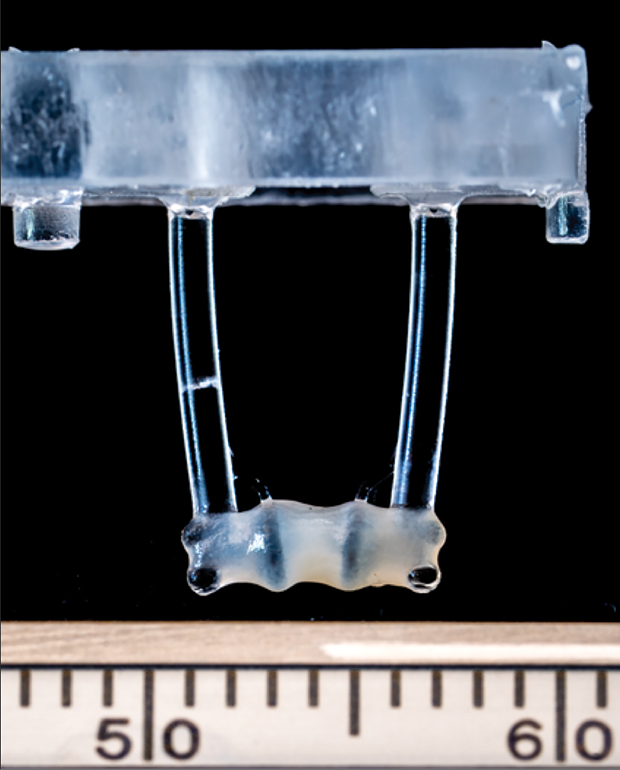
Groundbreaking work on replacement heart tissue is under way in a project led by Prof. Sian Harding at the National Heart and Lung Institute, Imperial College London, and funded by the British Heart Foundation (Figure 5, right). This project grew a 2 × 3-cm piece of durable, beating heart tissue from a patient’s stem cells, and is testing it as a replacement for dead heart muscle following a heart attack [6]. In essence, she said, “The idea is to actually bring the heart back to what it was before the infarction.”
For this work, Harding and her group began producing cardiac myocytes from embryonic stem cells, and then graduated to induced pluripotent stem cells. Her lab, which she described as “an ordinary academic lab,” is able to produce 60–70 million cardiac myocytes per week from iPSCs. This capability opened the possibility of using a patient’s own skin cells to generate iPSCs and consequently cardiac myocytes, which her group then set out to make into a tissue patch that the patient’s body would accept without rejection.
To get to that point, Harding’s group collaborated with researchers at the Universitätsklinikum Hamburg-Eppendorf (UKE), Hamburg, Germany, to convert the mass of individual myocytes, each of which has its own pacemaker, into strong cardiac tissue. “To do that, we suspended the cells in a soft fibrin hydrogel and added a bit of thrombin, which condensed (the mass) into a smaller space,” Harding explained. The cells not only connected up quite naturally, but they began beating as one. “Once we had that, we suspended the gel between two bendable silicon posts, so that when the tissue beats, it pulls the posts together,” she said, noting that this “exercise” caused the tissue to grow bigger, stronger, and thicker. “We ended up with a patch that is quite durable, is strongly beating in the dish, and is ready to be implanted,” she reported.
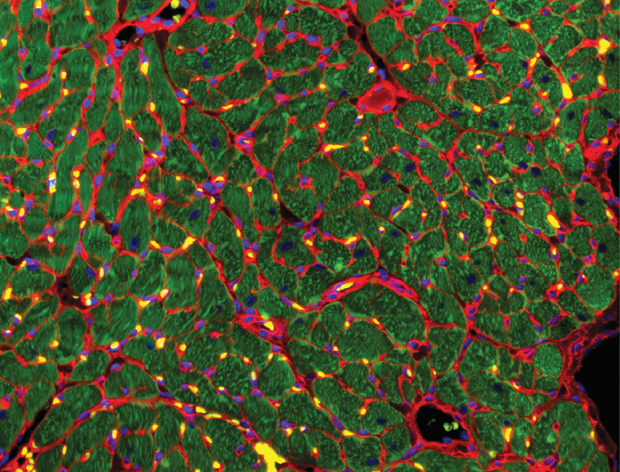
That led to the next phase of the project: successfully suturing the human heart patch onto a rabbit heart [7] to see how the two worked together. “We’ve been able to show various things. One is that the patch stays there, so over weeks to months, you can still detect the human muscle. You can also see blood vessels from the rabbit heart growing into all parts of the patch, so blood is going from the rabbit into the EHT, and you can see that the EHT is still beating,” she described (Figure 6). “Most importantly, when we produced an infarction in the rabbit heart and we put this patch on, the heart recovered in a month and its function went back to what it was at the beginning,” she said. “It actually works.”

Harding is now meeting with cardiac surgeons to explore the patch’s applicability in the clinic. Her group is also studying why researchers have found that pluripotent heart cells produce arrhythmias when they are directly injected into heart tissue, but do not have this issue when they are constructed into an engineered patch and laid onto the heart.
From here, Harding’s group is continuing the animal research as a prelude to the first human trials, and concurrently working with the U.K.’s Gene and Cell Therapy Catapult, a government-established organization to help industry and academics move new therapies from the lab to the clinic. She remarked, “I would hope that we might get the first patches into the clinic in two to three years if everything goes very well with the regulatory authorities.”
Heart, Heal Thyself
The patches require about six months’ time to prepare from the initial gathering of patient cells to the clinical implantation, “so we have to have the kind of patient who is going to be around and stable for that period,” Harding said. An alternative is to have generic patches made, match them as well as possible to the patient, and then administer immunosuppression so the patient doesn’t reject them, and she is looking into that option as well.
Another avenue for heart-attack repair revolves around introducing only the cardiac cells’ secreted factors—and not the cells themselves—as a way to stimulate the protection and regeneration of heart tissue. Several groups, including those of Harding and Vunjak-Novakovic, are looking into this possibility.
Vunjak-Novakovic’s group is investigating the tiny extracellular vesicles, exosomes, that are “loaded with cardiogenic, vasculogenic, and cardioprotective factors,” she said (Figure 7). “The idea is to use the cells that you would normally use to make a cardiac patch, and you culture these cells, let them secrete exosomes that are only 100 nm in diameter, physically collect them, and give them as a medicine either by catheter or intravenous injection.”
This approach would therefore “use the power of cells without using the cells themselves,” Vunjak-Novakovic said [8]. In addition, the factors would not necessarily have to come from engineered cells derived from the same patient. For instance, a blood sample from a healthy person would be enough to derive stem cells and use these cells to make millions of cardiomyocytes, which could then be essentially farmed for their exosome-secreted factors. “You could have these factors on the shelf, and you could give them to any patient, any time,” she said.
While this area is still under development, Vunjak-Novakovic is very optimistic. “I think it is potentially huge because if you just have a solution of these factors, you can give it to the patient right away after having a myocardial infarction; and there is no surgery if we succeed in developing administration routes by injection. So there is a lot of excitement about the use of exosomes as a parallel effort to the development of engineered cardiac tissue.” She added, “It’s super-promising and could be a new direction for heart treatment.”
Regardless of whether the path to cardiac care and repair routes is through an organ-on-a-chip, a patch, or exosome factors, Vunjak-Novakovic said the best results will necessarily come from collaborative efforts across disciplines. She remarked, “We are stepping out of our zones of comfort and talking to clinicians, stem-cell scientists, materials scientists, and others. Working together on a team that is truly interdisciplinary—this is how we can make progress. Today’s most exciting science is happening at the interfaces of disciplines.”
References
- J. Paez-Mayorga et al., “Bioreactors for cardiac tissue engineering,” Adv. Healthcare Mater., vol. 8, no. 7, Apr. 2019, Art. no. e1701504.
- Y. S. Zhang et al., “3D bioprinting for tissue and organ fabrication,” Ann. Biomed. Eng., vol. 45, no. 1, pp. 148–163, Jan. 2017.
- K. Ronaldson-Bouchard et al., “Advanced maturation of human cardiac tissue grown from pluripotent stem cells,” Nature, vol. 556, no. 7700, pp. 239–243, Apr. 2018.
- K. Ronaldson-Bouchard and G. Vunjak-Novakovic, “Organs-on-a-chip: A fast track for engineered human tissues in drug development,” Cell Stem Cell, vol. 22, no. 3, pp. 310–324, Mar. 2018.
- T. Chen and G. Vunjak-Novakovic, “Human tissue engineered model of myocardial ischemia-reperfusion injury,” Tissue Eng., vol. 25, nos. 9–10, pp. 711–724, May 2019.
- M. Kapnisi et al., “Auxetic cardiac patches with tunable mechanical and conductive properties toward treating myocardial infarction,” Adv. Func. Mater., vol. 28, no. 21, May 2018.
- R. Jabbour et al., “Development and preclinical testing of a large heart muscle patch,” Heart, vol. 105, pp. A157–A158, 2019.
- B. Liu et al., “Cardiac recovery via extended cellfree delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells,” Nature Biomed. Eng., vol. 2, no. 5, pp. 293–303, May 2018.