Boosting the body’s healing processes with bio-inspired wound care technology
The human body is miraculous in its capacity to heal but it can always use a little help. Wound care traditionally consists of little more than a protective barrier, possibly with an antibacterial agent, to cover the damage while the body works its magic. Now, a new technology has opened up a more active approach to encouraging healing.
Traction force-activated payloads (TrAPs) are a new material inspired by biological healing processes common to living organisms from sea sponges to humans, in which cell movement plays a vital part. Developed by a team led by Dr. Ben Almquist of the Faculty of Engineering’s Department of Bioengineering at Imperial College London (Figure 1), TrAPs can be easily added to existing wound care materials, where they actively interact with living tissue to promote the body’s own processes and deliver better outcomes for the patient.
“I was involved in the development of materials that interact with biological systems over time, especially for the delivery of therapeutic proteins in wound healing and that led me to look at the healing process as a biological system that evolves over time,” explains Almquist. “First, the body stops the bleeding, then it removes the damaged tissue, then it repairs the remaining tissue,” he adds. “We wanted to use materials to nudge it towards a more regenerative outcome. To do this you need to dance along with the body’s own processes, which requires materials that interact with the body over time instead of traditionally passive materials.”
Those passive materials—the simple wound dressings with antimicrobial agents—support the healing process and limit infection, but do not greatly improve the outcome in the way that Almquist envisaged. So, he started to consider different options. The first was a polymer that dissolves over time, releasing therapeutic agents. The major problem with this approach is that it, in order to synchronize the delivery of the agents at the right moment, assumptions must be made about the average times required for each stage of healing.

“The challenge was how to design smart materials that respond to the right stage of tissue repair at any given time,” says Almquist. “We started with proteins that could be passively released from a wound dressing but we wanted to take it into three dimensions. We wanted to pattern proteins in three dimensions that could be released by specific cells on demand.”
From passive to active
Advances in wound care have given us many new materials to aid healing. For example, implanted scaffolds can help to repair broken bones and collagen sponges can be used to treat burns and pressure sores. Until now, however, little has been done to actively support and encourage the process of tissue repair. Going back to basics, Almquist examined how the body switches from one stage of healing to another.
“We started out by looking closely at how wounds heal,” he explains. “One process of great interest occurs when the soft, squishy scaffold laid down by our body early in wound healing stiffens over time. A protein lies passively in the squishy scaffold until the scaffold stiffens, which then allows the protein to be activated and tell the cells to pull the edges of the wound together.”
“We started to wonder whether we could embed signals in materials and trigger them with mechanical stimuli. This is a natural process that goes back to sea sponges and sea urchins, so there is clearly an advantage to having these latent proteins present to aid wound repair. The question was whether we could turn this into an active treatment.” Almquist’s team saw that embedding and then activating proteins to send instructions to the body to repair tissue, then using that principle to deliver therapeutic proteins at the right time, could ultimately improve patient outcomes (Figure 2). It could, in fact, lead to the effective treatments of wounds that otherwise do not heal or heal very poorly.
A prime example is a diabetic foot wound. Higher blood glucose levels may prevent nutrients and oxygen from energizing cells, increase inflammation, or impair the function of the immune system. This is why patients with diabetes are 15 times more likely to have amputations as a result of foot wounds or ulcers. Almquist believes jump-starting healing in the skin or the bone could significantly improve that statistic.
TrAPs involve folding segments of deoxyribonucleic acid (DNA) into three-dimensional shapes (Figure 3). These are known as aptamers, which cling to proteins. A customizable handle is attached to the aptamer. Cells can attach to one end of this handle, while the other connects to collagen or another type of scaffold within the wound. It is the movement of cells through the tissue that pulls on the aptamers, causing them to unfold, hence the use of the term “traction force-activated” in the name. Once unfolded, the aptamer releases proteins that promote healing by instructing cells to grow.

The next step was to customize the handles, so that different types of cells can activate the aptamer and release proteins that are tailored to the stage of healing at which those cells appear. That is how TrAPs are synchronized with the body’s own healing process. “Essentially, TrAPs deliver growth factors that tell cells what to do,” Almquist says. “They activate and co-ordinate the repair process.”
Adapting antibody research
TrAPs technology builds heavily on the development of aptamers, which have emerged as an important tool in both research and clinical use. Aptamers are oligonucleotide (short nucleic acid polymers) or peptide molecules that bind to a specific target molecule. They consist of short, chemically synthesized, single-stranded ribonucleic acid (RNA) or DNA oligonucleotides that are folded into three-dimensional structures. They bind to their target in a manner that is similar to the interaction between antibodies and antigens.
Though similar to antibodies, aptamers have a significantly lower molecular weight, which enables them to more effectively reach the target tissue. Furthermore, they may be less toxic than antibodies. A relatively new class of agents for diagnostic and therapeutic purposes, aptamers can be cost-effectively synthesized in large quantities.
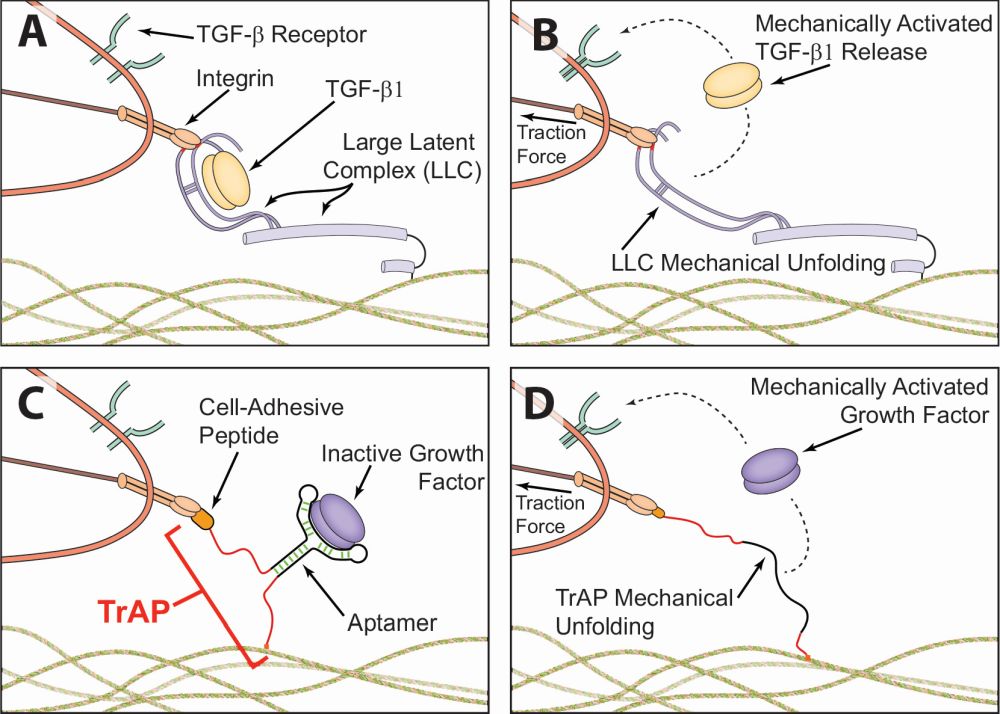
“There are many companies interested in the development of aptamers, which can bind to specific proteins, and they are approved for clinical use,” says Almquist. “We can leverage aptamer technology to adapt TrAPs to any protein of interest. TrAPs fold a single strand of RNA or DNA into a three-dimensional structure and, if you pull on it, it loses this three-dimensional shape, which eliminates the affinity for the protein.”
There exists the potential to encode a material that does not tell a protein to do its job until the aptamer is unfolded, at which point the protein would become active. Macugen, which was the first drug to treat macular degeneracy disease approved by the Food and Drug Administration (FDA), is based on aptamer technology, which could also assist the treatment of diabetes, blood clots, and cancer.
“Aptamers were developed as alternatives to antibodies and they can be used to inhibit, for instance, the over-expression of a protein that stimulates the growth of blood vessels in the eye, which can lead to blindness,” Almquist continues. “They were developed for treating pathological conditions but with TrAPs they can be used in therapeutic treatments not to inhibit but to promote blood vessel growth.”
An opportunity for better outcomes
The initial idea of an active approach to wound care has opened a door to a multitude of applications for TrAPs. As they can be triggered by different types of cells, they could be used not only to improve outcomes in skin and muscle repair, but also in the treatment of fractured bones, diabetic foot ulcers, scar tissue that develops after a heart attack, and many more applications. There is already interest in using TrAPs in the study of diseases, as well research into stem cells and tissue development.
“Skin and wound repair are obvious uses but we are also working on bone repair,” remarks Almquist. “TrAPs could be used in fractures when bones don’t fuse, or maxillofacial and dental applications such as repairing the jaw. In the future, they could be adapted for nerve injuries or potentially treating the damage caused by strokes.”
After five years of slow and steady development, TrAPs are ready for the next stage, which will see production scaled up to the industrial level. TrAPs are relatively simple to make and they are entirely synthetic, which means they can be recreated in different laboratories, quickly and in large quantities.
“The idea seems intuitive and I was surprised it had not been tried before,” Almquist says. “When we tried it, it worked just as we expected it to, so we were happily surprised. Now, we are optimizing the design for different applications, but many processes and components are already there because we are adapting aptamer technology.”
The most ingenious ideas often seem simple in retrospect, and there is great beauty in simplicity. In the case of TrAPs, the ability to scale up the production of aptamers is a distinct advantage, and there is no great complexity in their manufacture. There is a clear path to large-scale production, so progress in preclinical testing on bone repair could rapidly progress towards another significant milestone.
The potential applications of TrAPs are growing in number each day. Among them, Almquist hopes that one of the first will be in front-line medicine, particularly in low-resource areas, where a treatment that is more regenerative than scar-forming in traumatic wounds would be of immense value. TrAPs will undoubtedly take protein therapies into places where they have never been used before. It is truly a simple idea that could usher in a new paradigm in wound repair.