Where options are few, new technology for cancer treatment helps to fill a void
In the United States alone, an estimated 700,000 people have been diagnosed and are living with a brain tumor, and it is estimated that approximately 84,000 people will receive a tumor diagnosis in 2021 [1]. Fortunately, the majority of these tumors will be benign; on average only 30% of all brain tumors are malignant. For patients with malignant tumors, the current five-year survival rate is 36% with an average 31% ten-year survival rate [2], but for those diagnosed with glioblastoma (GBM)—one of the most deadly and treatment-resistant cancers—the patient survival rate falls to a low 7.2% and the median life-span after diagnosis is only eight months. GBM is the most common primary malignant brain tumor, even as an increasing numbers of cancer patients are diagnosed with brain metastases (secondary brain tumors), where the cancer has traveled to the brain from another part of the body.
“When it comes to the brain, even with benign tumors people can die because the tumors grow confined in the brain,” says Melvin Field, M.D., a neurosurgeon and medical director for the AdventHealth Minimally Invasive Brain Surgery program, as well as an associate professor of neurosurgery at the University of Central Florida (UCF) College of Medicine. “And with GBM, the tumors are highly malignant, rapidly progressing, and historically, somewhat limited when it comes to treatment options.”
Overall, these statistics paint a grim picture, expounded by the fact that there are few Food and Drug Administration (FDA)-approved treatments available to address brain cancer. In most cases, surgery and radiation have remained the primary options. But even within the limitations of these approaches, the toolbox for clinicians is slowly growing and the ways in which researchers and surgeons think about and treat brain tumors has changed.
“Surgery is not necessarily curative because microscopic cells are left in the brain,” Field explains. What is needed, he says, are ways to identify these small areas of cancerous cells and prevent them from growing back. With advances in areas such as molecular phenotyping, research is uncovering more information about the tumors themselves and what genes are causing them to spread. In fact, a new frontier in cancer treatment aided by molecular medicine is emerging. This includes approaches such as chemiluminescence-guided cancer therapy, where small areas of tumor cells can be identified using light [3]. At the same time, a better understanding of the unique genetic traits of cancer is opening pathways to more individualized treatment combinations.
As tools for treatment increasingly become more precise and individualized, the chances for improved outcomes for the patient also increases. “We are moving away from treating the entire brain to a more focused approach,” Field says. For example, the use of standard radiation—essentially whole brain radiation—has been the standard of care for many years and has proven somewhat effective. But that type of whole scale approach generally leads to a number of side effects for the patient over time, particularly for those who survive for two or more years. “Radiation is merely a form of energy, and using it to disrupt the replication process of [cancerous] cells also affects other replicating cells,” Field says.
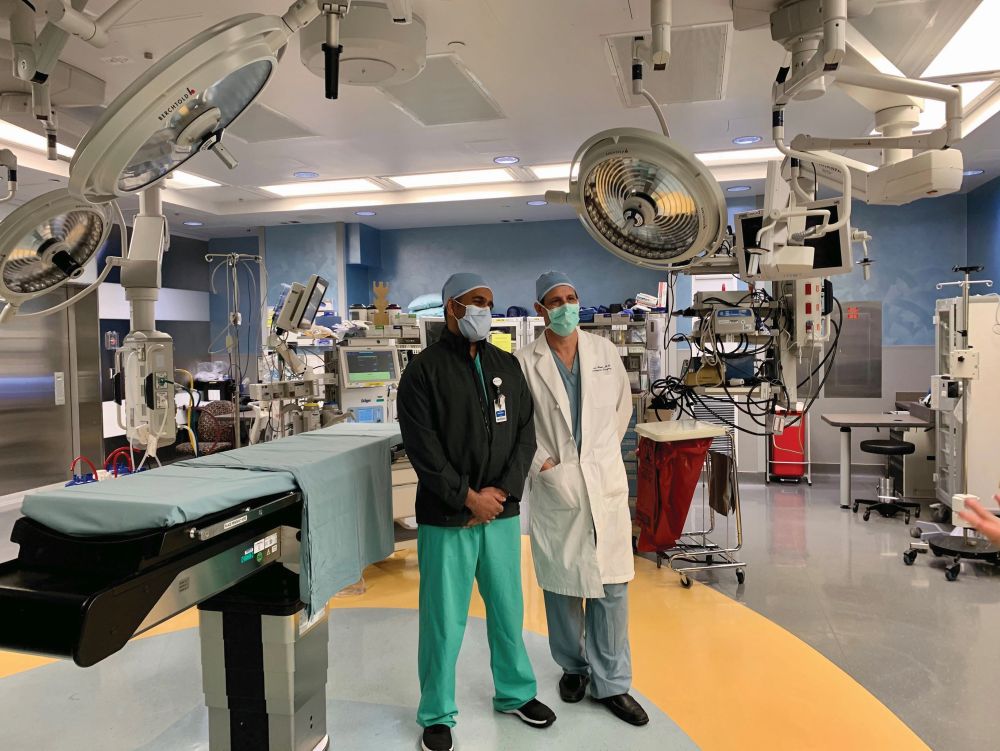
Refining the surgical approach through advanced technology options offers a level of exactitude that helps surgeons direct radiation away from the healthy tissue and target the tumor more directly. This type of targeted therapy has the potential to cure lower grade tumors and may lead to better long-term survival overall. “As a field, we now have a variety of options for treatment, and we will never complain when someone offers us a new tool,” says Imran Mohiuddin, M.D., Ph.D., a radiation oncologist at AdventHealth (Figure 1).
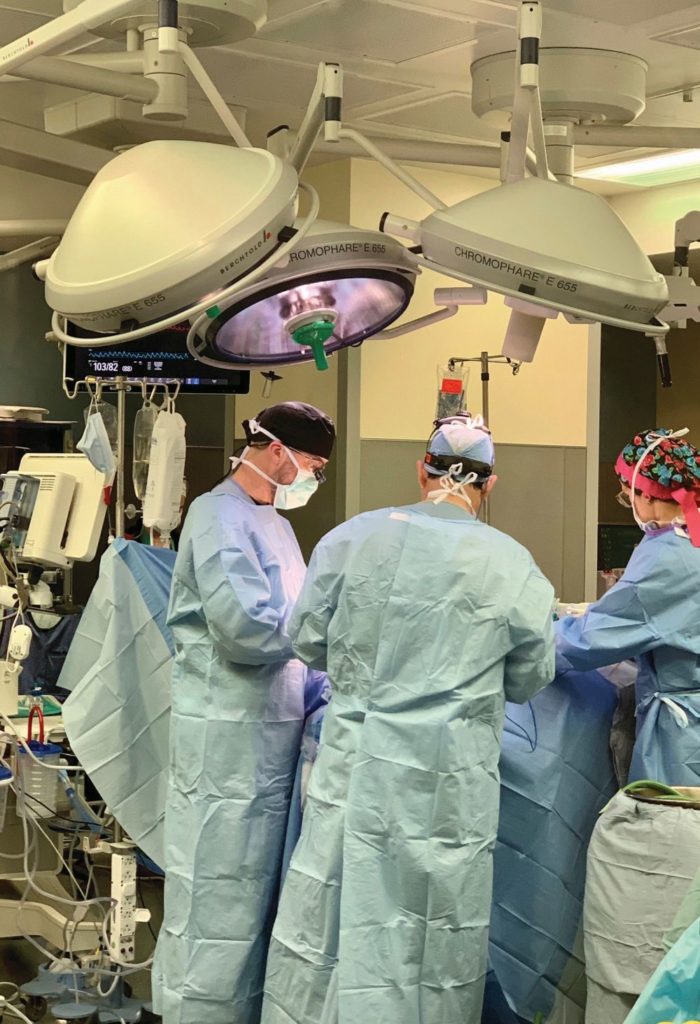
One such device recently available is GammaTile, which allows for the application of targeted radiation delivery at the time of surgery. GammaTile is comprised of a biodegradable composite—a collagen matrix—embedded with radioactive seeds to create Surgically Targeted Radiation Therapy (StaRT). During surgery, the cancerous tumor cells are first removed and then the square tiles are implanted in the surgical cavity (Figure 2). Over a period of about six weeks, the radioactive seeds emit a focused high energy in the surgical area in order to target any remaining microscopic cancerous cells. The therapeutic dose is most concentrated in the first ten days after implantation in order to minimize replication of residual tumor cells.
One benefit here is that damage to healthy cells is minimized. “There is a sweet spot of placement for the radiation,” Mohiuddin says. Because the seeds are insulated with collagen, they do not come in direct contact with the tissue. “The structural buffer of collagen outlasts the radiation, so even though this is a permanent implant, the seeds eventually become inert and the collagen is absorbed into the body.”
Another benefit is that with this type of approach, radiation therapy begins at the time of surgery rather than a later date. Typically, a patient would need to wait until the surgery has healed before undergoing radiation treatment. But when using GammaTile technology, “we don’t have to worry significantly as risk is lessened because of the tight radius of contact,” Mohiuddin says. This is especially true for those who have already undergone other radiation treatments, and for patients who have run out of other options due to complications or regrowth. “We need to employ a multi-modality approach for some tumors,” he adds. “Tumors don’t always behave as expected, and we now have an option that is safe, which also can be effective.”
The goal for treatment is to improve chances of survival by controlling the growth of the tumor. “Ultimately, for some people using this technology may equate to a cure when cure wasn’t possible, and at this point it may help patients live longer,” Mohiuddin says.
In the coming decade, the technology tools available to treat cancer likely will have multiplied and evolved. New approaches that employ ultrasound technology are under clinical trial, and other precision radiation treatments are on the horizon. Although these treatments still rely on forms of energy, at some point genetic information and understanding of brain tumors will hopefully enable prevention rather than treatment. “In the next five to ten years, vaccines that target the proteins on tumors will be available,” Field says, “and use of mRNA options will likely transform cancer treatment.”
For those who work with patients, these developments will be a game-changer. “As neurosurgeons, basically we are very fancy carpenters; we have a toolbox to create and fix things,” Field explains. “Historically, we have broken down a wall and removed things, and we replaced the pipes when they were clogged, becoming more like plumbers. Eventually we will become interventionists, using other therapies that are instilled and spread into a tumor. The way we think about surgery then will be completely different, and we will become less a butcher and more a sculptor.”
Imagining a future with new life-saving approaches for brain cancer may soon become reality. “I think the holy grail is some kind of treatment that cures a person from a biologic level. If we could cure without radiation at all that would be ideal,” Mohiuddin says. For the thousands of people diagnosed with brain cancer every year, this future can’t come soon enough. Until then, the addition of new tools such as GammaTile offer some hope for living with brain cancer.
References
- [Online]. Available: https://braintumor.org
- [Online]. Available: https://www.cancer.net
- D. Mao et al., “Chemiluminescence-guided cancer therapy using a chemiexcited photosensitizer,” Chem, vol. 3, no. 6, pp. 991–1007, Dec. 14, 2017, doi: 10.1016/j.chempr.2017.10.002.