Paralysis, whether caused by spinal cord injury, neurodegenerative disease, or other factors, poses a host of issues for patients. These include not just the inability to move parts of their bodies but potential problems with communication and bladder control as well. Fortunately, the last decade has seen promising technology advances to address these concerns. Although most are still in the research stage, these technologies—including brain–computer interfaces (BCIs), exoskeletons, and robotics—may one day improve the lives of people with paralysis.
Communicating Through Brain Waves
Some people who have lost the ability to talk use devices that allow them to select letters on a screen using head or eye movements. Renowned physicist Stephen Hawking, for instance, communicates this way. But BCIs (also known as brain–machine interfaces) increasingly show the potential to allow communication in a more intuitive way. BCIs sense brain activity through electrodes that either sit on the scalp or are implanted in the brain. They work by reading brain activity and turning it into a signal that can then be used to direct something in the environment, such as a cursor on a screen. In this way, even someone having no voluntary movements can communicate and perform various other actions.
One leading approach in this area is the BrainGate Neural Interface System. BrainGate constitutes a consortium of neurologists, neuroscientists, clinicians, engineers, and others at Brown, Stanford, and Case Western Reserve universities, along with their affiliated hospitals. In 2005, BrainGate researchers implanted the first BCI into a human patient paralyzed from the neck down. In systems like this, doctors implant a sensor on the surface of the brain’s motor cortex, the part of the brain responsible for voluntary movement. Then, when the user imagines moving a cursor or prosthetic limb, the sensor transmits the electrical signal to a computer that decodes these signals into action.
Recently, the BrainGate team built a new BCI that allows paralyzed people to type by thought alone, at a faster rate and more accurately than with previous systems. The team implanted a tiny silicon chip—just over one-sixth of an inch square—from which protruded 100 electrodes that penetrated the brain and could tap into individual brain cells in the motor cortex. The array recorded the electrical signals from nerve cells that fired as the patient thought of performing a motion, such as moving the right hand. Then software decoded the signal and converted it into a command for the computer cursor.

BCIs may even allow patients with no voluntary movement at all to communicate. Led by Niels Birbaumer (Figure 1) of the Wyss Center for Bio and Neuroengineering in Geneva, Switzerland, scientists implanted BCIs in four patients with locked-in syndrome. This syndrome can arise as a result of amyotrophic lateral sclerosis (ALS), a progressive neurodegenerative disorder, and causes complete paralysis except for simple eye movement. Some patients eventually lose even their ability to blink, cutting off all communication with the outside world.
Rather than an implanted BCI, Birbaumer and his colleagues used a BCI that fit on the patient’s head like a swimming cap (Figure 2). It measures changes in electrical activity and blood flow in the brain and enables patients to answer yes or no questions by modulating their thoughts. This allows doctors to ask locked-in patients questions about their quality of life and desire to live. Three of the four patients answered “yes” when asked if they were happy and replied positively to the statement “I love to live.”
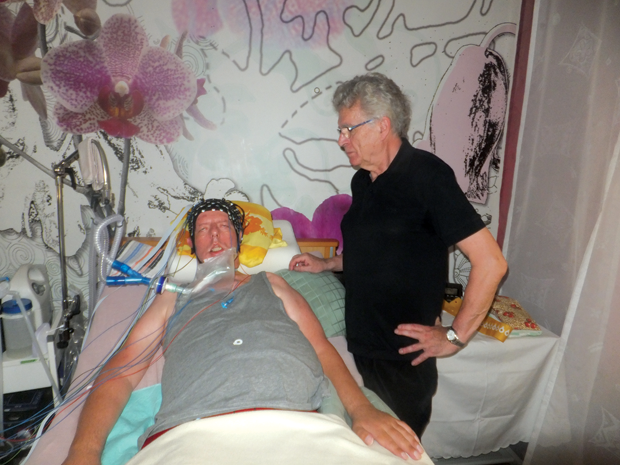
Birbaumer says that his team’s task for the next few years is to develop a BCI that is even more user friendly and flexible. “All the patients who have ALS and are completely locked in can use this system for yes-and-no questions,” says Birbaumer. “We are trying to do more flexible communication with these patients, something that allows them to select letters or words with brain activity.” This might require an implanted BCI, rather than one that sits on the scalp like the BCI used in this study. Birbaumer is hoping to be able to publish the results of these changes to the system within the next two years. Although recording from inside the brain requires surgery, many patients are willing to go under the knife if the result enables them to communicate or otherwise interact with their environment.
Moving Again with Exoskeletons
Researchers are using BCIs to control more than just typing systems; they are also making remarkable progress in connecting BCIs to prosthetic limbs and exoskeletons. Such systems can restore the freedom of movement that stroke and spinal cord patients have lost, and they may even aid in rehabilitation.
In a highly anticipated demonstration at the 2014 World Cup opening ceremony in Brazil, a young man, paralyzed from the chest down, delivered the opening kick. He used a BCI consisting of a cap of electrodes that recorded his brain signals and sent them to a laptop-sized computer worn on his back, triggering his lower-limb exoskeleton to execute the kick.
The demonstration, seen by about 1.2 billion people, came out of the Walk Again Project (WAP), a nonprofit, international research consortium. Two years later, WAP published its first clinical report. It included some surprising results: a group of spinal cord injury patients regained some small but significant sensation and movement in their lower limbs after many months of training with a BCI hooked up to a motorized exoskeleton. The participants are not anywhere near being able to walk unassisted as yet, but some can voluntarily move muscles in their legs and have improved bladder and bowel control.

Duke University’s Miguel Nicolelis (Figure 3, right), lead author of the report, says his team’s original goal was to help paralyzed patients move around with the assistance of mind-controlled exoskeletons. The eight patients who participated in the study went through three levels of training with a BCI, which consisted of a cap full of electrodes that fit over their scalps. In the first phase, electrical brain signals picked up by the electrodes were translated into digital commands that controlled a virtual avatar of the patient. Patients wore an Oculus Rift virtual reality display; by imagining the movements of their own bodies, they could make their avatars walk (Figure 4). In the second phase of the protocol, patients moved on to using their brain signals to control a robotic walker system on a treadmill. Finally, the patients operated the same kind of brain-controlled, motorized exoskeleton demonstrated at the 2014 World Cup (Figure 5). “Not only were these patients able to use BCIs to control exoskeletons that allowed them to walk, but after several months of training we noticed that some patients showed signs of neurological improvement that nobody had seen before,” says Nicolelis.

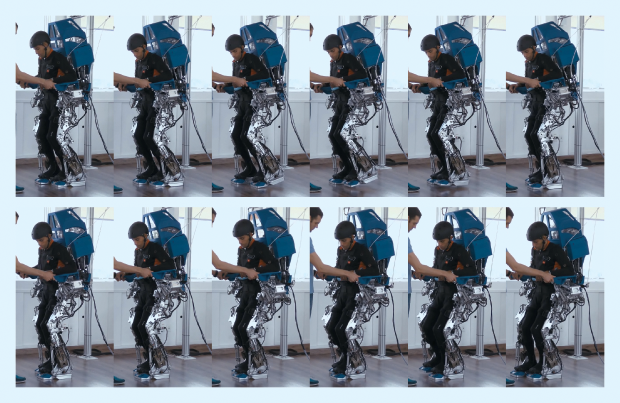
One key to the WAP protocol’s success is that it offers both visual and tactile feedback to patients; they can see and feel when their nerve signals are responsible for the movement of an artificial limb. Tactile feedback is provided through a specially designed long-sleeved shirt that vibrates the patient’s arms whenever his or her avatar or exoskeleton lands a step. The combination of corroborating visual and tactile feedback creates a realistic walking illusion for the patients when they control the virtual avatar or the robotic exoskeleton.
Nicolelis and his colleagues suspect their protocol promoted brain reorganization and recharged dormant neural circuits that may have survived the original spinal injury. “I think we triggered a process of plastic, functional reorganization in the brain,” explains Nicolelis. “By activating the brains of these patients with this training, we may have allowed sensory and motor messages to be rerouted through these surviving nerves that were silent for years.”
Nicolelis feels the WAP protocol could one day help paraplegic patients by upgrading BCIs from an assistive technology to a new therapeutic approach for spinal cord injury rehabilitation. The first two stages—the virtual reality and treadmill systems—could easily be adopted, but it will be more difficult to bring expensive custom exoskeletons to a large number of patients. Future research will explore how each stage contributes to recovery and help determine whether all three stages are necessary.
An Invasive BCI Without Surgery
Many BCI systems rely on an invasive approach, in which electrodes are implanted surgically into the brain. Although often effective, open-brain surgery comes with risks to the patient. Researchers from the University of Melbourne, Australia, recently developed a new matchstick-sized device that can be implanted into the brain through blood vessels, bypassing the need for invasive surgery (see also Jim Banks’s “Moving Objects with Your Mind”). Dubbed a stentrode (a combination of stent and electrode), the technology uses the blood vessels within the brain as a highway to reach the motor cortex, without the need to open a hole in the skull. “We have built our system on the back of other technologies, like stents for aneurysms and microcatheters,” says Thomas Oxley, one of the lead developers of the stentrode.
The device is delivered through a small catheter; when in position over the motor cortex, the catheter is removed, deploying the stentrode. The stentrode then expands and attaches to the walls of the blood vessel, where it can record the activity of nearby neurons and translate these signals into commands that could be used to control an exoskeleton.
Oxley and his colleagues tested the stentrode in the brains of healthy sheep for a period of six months, demonstrating that the technology is safe and effective. The researchers’ ultimate goal is to implant stentrodes in human patients and connect them to exoskeletons. They are progressing toward human clinical trials in the next year.
“We’ve evolved from a research group into a startup company,” explains Oxley. “We intend to recruit fewer than five patients with severe paralysis and demonstrate that our system is safely implantable and chronically viable, and that we can record activity that can be used to achieve basic movement and communication control of assistive technologies.” If the trials succeed Oxley believes the technology could become available in five to seven years.
More Realistic Robotic Parts

When it comes to robotic body parts, a major issue is that they don’t look, feel, or behave quite like the real thing. Most robotic body parts used today are rigid and have limited range of motion. But scientists led by Erik Engeberg at Florida Atlantic University, Boca Raton, have designed a new type of soft bionic finger that looks, feels, and works like the ones on your hand. Engeberg and his colleagues (Figure 6) developed and tested this robotic finger using a three-dimensional (3-D) model of a human finger, shape memory alloy, a 3-D printer, and a unique thermal training technique.
First, the researchers took a model of a real human finger and used a 3-D printer to create fleshlike inner and outer molds. Inside, they placed two actuators made of shape memory alloys, which can remember and return to their original shape when heated. The extensor actuator straightened up when heated, whereas the flexor actuator curved. “When we alternately heated and cooled the two different actuators, we got an antagonistic motion, which is somewhat similar to the kind of actuation you see in biology,” says Engeberg. “For instance, wrists, fingers, arms, and legs typically use an antagonistic actuation of muscles to cause motion at the joint.” The result of this thermomechanical training is a robotic finger that can mimic the motions of a human finger, such as flexing and extending.
There are still challenges to work out, including the lengthy amount of time it takes for the actuators to cool and return to their natural shape. That’s why Engeberg and his colleagues have decided to use the technology for underwater robotics first, as the water will provide a rapidly cooling environment. However, the researchers imagine future applications for human patients. “Due to its light weight, dexterity, and strength, our design could be incredibly useful in wearable applications,” claims Engeberg. “Ultimately, our robotic finger could be adapted for use as a prosthetic device.”
Better Bladder Control with Robotics
Of all the issues that spinal cord injury patients must deal with, bladder control is near the top of the list. People with spinal cord injury have no bladder sensation and no control over the bladder emptying. Currently, doctors paralyze the bladder with botulinum toxin, and patients must catheterize themselves several times a day to empty their bladders. This takes care of the problem of unwanted bladder emptying but can cause infections and scarring in the urethra.

James Fawcett, head of the John van Geest Center for Brain Repair at the University of Cambridge (Figure 7, right), is developing a device to address these problems. It is based on the Brindley device, an implant to which an external stimulator is applied manually, causing the bladder to contract and empty. The Brindley device is not currently used very much because it requires a surgical operation and involves severing sensory nerves from the pelvis into the spinal cord, which weakens the pelvic muscles and results in a loss of sexual function.
Fawcett and his colleagues are working on a biorobotic version of the Brindley device that can read signals from the sensory nerves in the pelvis, rather than cutting them. “These signals would tell the patient how full the bladder is, stop the bladder from emptying itself spontaneously, and allow them to use the electronics to empty their bladders,” Fawcett explains.
So far, Fawcett and his colleagues have trialed a robotic Brindley device in approximately 50 dogs. Spinal cord injuries are common in breeds with longer spines, like dachshunds, and as the result of road accidents. “You put a pair of wheels under under the back legs, and the dog will paddle around quite happily with a paralyzed rear end,” says Fawcett. “The problem is they have a paralyzed bladder, so they paddle around dribbling urine.” The dog version of the robotic Brindley device has been extremely successful; some devices have been implanted for over a year.
This technology has applications beyond just bladder control: Fawcett originally envisioned it as enabling patients with paralysis or missing limbs to control robotic prostheses. “If you damage a nerve in a limb, the damaged nerve fibers will regenerate a bit, but only for small distances, and they tend to regenerate randomly and reinnervate the wrong muscles,” according to Fawcett. “Our long-term aim is to take information from the nerve above an injury and use it to stimulate the limb below it.”
New technologies such as BCIs, exoskeletons, and robotics have the potential to allow paralyzed patients more control and freedom in their daily lives. Researchers worldwide are working toward making such technologies more powerful, user friendly, and efficient. In the coming years, they hope these new technologies will make the leap from the lab into patients’ homes.