As a growing epidemic of opioid abuse in the United States can attest, pain, and how to treat it effectively and without serious side effects, is one of the foremost challenges in medicine today.
More than 100 million Americans have pain that lasts for weeks to years. It’s the main reason people seek out doctors, costing US$600 billion a year in health care spending. The burden of pain is felt by patients, families, friends, and health care providers. In an effort to blunt pain, some patients end up addicted to powerful and dangerous narcotics. Even worse, some die from overdose of these painkillers.
Spurred on by this grim reality, researchers are looking to new biomedical technologies for pain management, continuing to refine older techniques such as neurostimulation while pushing into new frontiers such as exploring how light delivered to certain neurons can quell pain and how manipulating the gut microbiome might dull sensation. While research is still in the early stages in some of these techniques, what is clear is that doctors may one day soon have many more weapons in their arsenal against pain than they presently do.
Zapping Pain Away
One of the more established techniques for controlling pain is neurostimulation, which has been used for pain management for more than 40 years, and which continues to intrigue scientists for its potential. It’s an approach that aims to change neural excitability and connectivity, altering the function of nerves that may be hypersensitized or damaged and continue sending pain signals long after an injury.

One of the most common forms of neurostimulation, spinal cord stimulation (SCS), involves placing a pacemaker-like electrical lead beneath the skin and along the spinal cord that is then connected to a pulse generator that sends mild current to the spine.
“SCS works in the spinal cord to change the way pain conduction goes from the spine to the brain,” says Timothy Deer of the Center for Pain Relief in Charleston, West Virginia, a center treating more than 3400 chronic pain patients each month. “Traditionally, it’s been used for neuropathic trunk and limb pain, pain from nerve damage in the spine or periphery.”
Deep brain stimulation (DBS) is another invasive neurostimulation technique, which involves surgical stimulation of the brain, usually the thalamus or motor cortex. DBS is a relatively well-established and effective treatment for movement disorders like Parkinson’s disease, but another potential use is for chronic pain conditions that do not respond to other treatments. For many patients with neuropathic pain, DBS has led to long-term improvement in pain, emotional well-being, and quality of life.

“We found an average pain reduction of 50%, with one in five patients reporting more than 80% improvement in pain,” says Alan Gray of the University of Sheffield in the UK. “We also found that functional ability, the degree to which patients are able to do day-to-day tasks, improved after surgery, as well as depression and anxiety symptoms” [1].
Researchers have explored transcranial direct current stimulation (tDCS), a non-invasive technique in which low intensity electric current is delivered to the brain using electrodes placed on the scalp, in a wide range of pain conditions, such as fibromyalgia, trigeminal neuralgia, migraine, and post-operative pain. The results have been promising, but mixed. Although most trials reported pain relief following tDCS, some studies had negative results. This is not altogether unexpected given the high variability in stimulation protocols, electrode placement, and patient populations, says Helena Knotkova, Director of Clinical Research and Analytics at the MJHS Institute for Innovation in Palliative Care, NY, and also at the Albert Einstein College of Medicine in New York .

“The trend is to make devices user-friendly and portable so they can be used by patients at home,” she says. “I have some ongoing studies with tDCS where the device has enhanced safety features and can be used at home, and the patient and clinical team communicate over video to see if the patient needs any assistance.”
“We have had neurostimulation for over 40 years, but in the last three years there has been more research and development than ever before,” says Deer. “That’s driven by improvements in technology, miniaturization of computer parts, and the demand for something other than opioids for pain relief. We’re in the golden age of neuromodulation now, and I think it will continue for a long time moving forward.”
Shining a Light on Pain Relief
Optogenetics has emerged over the last ten years as a transformative technology for controlling and understanding animal behavior. By genetically changing neurons to turn on or off when exposed to light, scientists can target specific neurons or neuronal circuits. It involves two parts: the modification of a cell’s DNA to express a light-sensitive protein, and a means to deliver light to that cell, typically accomplished with an implanted fiber-optic cable. With optogenetics, scientists can tell neurons to fire or block them from firing with a flash of light. Traditional methods of studying pain in animals cause tissue damage and activate a slew of cells in addition to the pain-sensing neurons of interest.

“Optogenetics is highly specific,” says Samarendra Mohanty of NanoScope Technologies, LLC, based in Arlington, Texas. “You can activate one type of neuron precisely and instantaneously.”
Scientists are beginning to use optogenetics to understand the cells and circuits involved in pain. There are also studies showing that pain can be turned on and off with light in animal models. Using either transgenic animals or gene therapy, researchers have created mice whose pain could be dialed up or down by shining light on them. In these experiments, light activated pain-sensing cells in the brain and elicited pain behaviors, like paw withdrawal, licking, jumping, and squeaking.
Mohanty and his colleagues have shown that optically stimulating a small area of the brain called the anterior cingulate cortex (ACC) reduces pain in laboratory mice (Figure 1, below). Previous evidence from humans and other animals had singled out the ACC as being important in perceiving pain. But existing electrode-based ACC stimulation is not specific, activating both excitatory and inhibitory neurons. “We have shown you can use optogenetics to reduce pain by specifically stimulating the inhibitory neurons of the ACC,” says Mohanty.

Right now this research is at the experimental stage and is only being used in animal models. But researchers such as Robert Gereau of Washington University in St. Louis, see the potential for using optogenetics for pain management in human patients. “The basic concepts have been demonstrated,” Gereau says. “Scientists have shown that we can suppress pain in animals using optogenetics. Now the long process begins to make sure this technology can be used safely in people.”

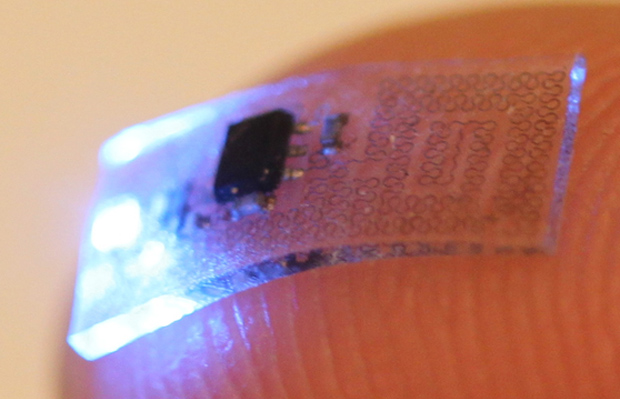
There are plenty of hurdles for an optogenetic therapy. One is the light delivery system. Optogenetic devices tend to be rigid, bulky, and tethered to a power source that can inhibit movement. Gereau was part of a team of researchers that developed miniaturized, flexible, wireless optogenetic devices that were implanted in mice [2]. The devices were soft and stretchy, so they moved with the animals, and were as small as your fingertip (Figure 2). The devices were implanted in mice that had been genetically engineered to have specific neurons respond to light (Figure 3). The researchers could control pain signals from the body and spinal cord of the mice before those signals reached the brain. This proof of concept could eventually lead to medical implants for people living with chronic pain.
The other major obstacle for an optogenetic treatment is getting light-sensitive proteins into the neurons of human patients. In rodent studies, scientists have used two strategies. The first, transgenics, involves animals that are bred to express light-sensitive proteins in specific neurons, and is not suitable for use in humans. The other method is gene therapy, in which a virus is used to shuttle a gene into a cell. However, much work needs to be done to confirm that such interventions would be safe over the long term in people.
“You need a gene therapy vector that would deliver a light-sensitive protein specifically to neurons involved in pain, and you would need to pair that with a device that would deliver light and allow you to control the activity of those cells,” says Gereau. “There are two parts of this therapy, both of which require extensive clinical trials and regulatory approval before they could be used in people.”

Several start-up companies are hoping to pursue optogenetics trials in patients within the next few years. Circuit Therapeutics, founded in 2010, wants to begin clinical trials of optogenetics to treat chronic pain, while Mohanty is leading the scientific team of NanoScope Technologies to develop prosthetics for optogenetic pain management. Circuit Therapeutics, based in Menlo Park, CA, was founded in 2010 by Stanford University researchers to treat neurological disorders with optogenetics. In 2015, the company won a US$2.7 million dollar contract from the Defense Advanced Research Projects Agency (DARPA) to pursue optogenetic therapies. The award will allow Circuit to expand its studies optimizing gene therapy and light delivery to nerves that control neuropathic pain. One reason the company chose neuropathic pain for its upcoming clinical trials is that the neurons affected by this pain are accessible, located on the outside of the spinal cord rather than the brain.
Gereau believes the development of implantable devices might come along relatively quickly, while the gene therapy component will be a bigger hurdle. “This is an exciting prospect for the treatment of pain,” he says. “There’s no way to know how close we are to using this in people, but the proof of principle experiments are there. We know, at least in theory, that pain management is possible using optogenetics.”
The Gut-Brain Connection
The human gut is home to a rich array of bacteria and other microorganisms, estimated at 10^13 – 10^14 microorganisms and composed of over a thousand species. In recent years, scientists have gathered mounting evidence that these bugs have a major impact on our well-being.
Gut microbiota have the ability to communicate back-and-forth with the brain via a pathway known as the gut-brain axis. The brain acts on gastrointestinal and immune functions that may modulate the composition of the gut microbiome, while gut microbes can influence neurological activity by producing an array of metabolic substances and neurotransmitters. A microbiome with the wrong species of bugs, or the wrong ratio of bugs (a condition called dysbiosis) may wreak havoc on a person’s health – in their gut and throughout the body. So what determines the makeup of one’s gut microbiome?
It starts at birth, and is influenced by factors like whether one is delivered vaginally or by C-section and whether a baby is breast-fed or formula-fed. A person’s gut microbiota is continually impacted throughout their lives by diet, age, antibiotic use, and lifestyle.
One type of pain thought to be affected by gut microbiota is visceral pain. This is any pain elicited from the organs, or viscera, of the abdominal cavity, explains Colin Rudolph, vice president of global medical affairs and chief medical officer at Mead Johnson Nutrition, a major manufacturer of infant formula based in Illinois. “Visceral pain is transmitted and felt differently than peripheral pain, such as muscle pain or a burn on your finger,” says Rudolph. “It tends to not be as specific in location and it generalizes across the abdominal cavity.”
Recently, Rudolph and his colleague Maciej Chichlowski reviewed the evidence connecting visceral pain and the intestinal microbiome in rodents. Studies show that exposure to stressors or treatment with antibiotics early in life both alter the types and abundance of bacteria found in the intestines, and these changes may be associated with increased visceral pain responses in adult animals. “Animals that are germ-free–mice delivered by C-section and raised in a completely germ-free environment–have a different sensitivity to pain,” says Chichlowski. “But the mechanisms underlying this increased sensitivity are not well known.”
There is also evidence that changes in gut microbiota are linked to visceral pain in humans. Patients with gastrointestinal disorders such as inflammatory bowel disease and irritable bowel syndrome show an altered gut microbiota profile. Chichlowski and Rudolph say it is possible that management of dysbiosis early in life could prevent the development of such conditions.
In another recent study, Chichlowski, Rudolph, and their colleagues looked at how treatment with probiotics affected visceral pain in rats. “Rats fed a probiotic diet had less visceral pain later in life, and this was accompanied by changes in some key brain neurotransmitters,” says Chichlowski [3].
The researchers caution that this is still very early research. The experiments with animal models cannot be directly applied to humans because the human microbiome is different from the rat microbiome. But it provides a proof of concept: that by changing the microbiome one can change pain responses. It is also becoming clear that gut microbes can affect the immune system, even in diseases outside of the gut. For instance, intestinal bacteria appear to play a role in rheumatoid arthritis, an autoimmune disease in which the body attacks the joints.
VeenaTaneja of the Mayo Clinic and colleagues discovered differences in the bacterial populations of mice bred to be genetically prone to rheumatoid arthritis. In mice genetically susceptible to the disease, a species of Clostridium bacteria dominated their gut microbiota. This strain of bacteria was uncommon in mice without arthritis. Taneja says that hormones and changes related to aging may further alter the gut immune system and contribute to inflammatory conditions in genetically susceptible individuals.
In another study, scientists found that people with rheumatoid arthritis had higher amounts of a bacterium called Prevotellacopri in their intestines than people without the disease. While evidence of the link between the microbiome and arthritis grows, the exact mechanism of action is not well known. Bacteria like Prevotellacopri might stimulate an immune reaction to cause inflammation and pain in the joints. Or their presence could crowd out beneficial microbes that might signal the immune system to take it easy.
Taneja thinks eventually it will be possible to treat diseases like rheumatoid arthritis by adjusting the microbiome. “The field is moving towards trying to figure out, for specific diseases, what good bacteria patients are lacking or what bad bacteria have grown out of control,” says Taneja. “We can then either target the bad bacteria or the good bacteria to balance the gut microbiome and tailor drug treatments to individual patients.”
But it’s not as simple as just taking any old probiotic. “You need to know which probiotic to use in what population and clinical situation,” says Rudolph. “Down the road, we might be able to do a quick analysis of a patient’s microbiome to see what components are missing or overactive and then give that patient an individualized prescription to bring it back into balance. But there’s years of research to be done before we can bring this into clinical practice.”
References
- Gray, A. M., Pounds-Cornish, E., Eccles, F. J. R., Aziz, T. Z., Green, A. L., and Scott, R. B. (2013). Deep brain stimulation as a treatment for neuropathic pain: A longitudinal study addressing neuropsychological outcomes. Journal of Pain 15(3): 283-292. doi:1016/j.pain.2013.11.003.
- Park, S. I., et al. (2015). Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nature Biotechnology 33: 1280-1286. doi:1038/nbt.3415
- Chichlowski, M. and Rudolph, C. (2015). Visceral pain and gastrointestinal microbiome. Journal of Neurogastroenterology and Motility 21(2): 172-181. doi:5056/jnm15025.