Using new approaches, researchers prime micro- and nanorobots to maneuver through the body to deliver targeted therapies
“The traditional way of delivering drugs has a very low efficiency. For instance, with solid tumors, drug delivery efficiency is reported to be lower than 1% [1], which means that 99% of the drug is elsewhere in the body causing side effects instead of actually fighting the cancer. This is where micro- and nanorobots can come into play, because they can swim or otherwise move to the target location in a controllable way. This is the hope.”—Tian Qiu, Ph.D., biomedical robotics developer
Futurists have long dreamed of the day when micro- and nanorobots will carry therapeutic drugs to the exact spot in the human body where they can do the most good. The body, however, is a crowded and dynamic place that is armed with a variety of seek-and-destroy defenses and highly effective barriers that present huge challenges to targeted micro- and nanorobot delivery.
To get past this hurdle, researchers are trying all sorts of tactics, and they are making headway. This includes three intriguing efforts that are taking very different approaches:
- one has devised hybrid microrobots, which incorporate living, motile organisms as the transport vehicles, and have deployed them in animal experiments to successfully treat pneumonia;
- a second is taking cues from living organisms to help micro- and nanorobots maneuver through dense tissue, perhaps even into the deep tissue of the brain; and
- a third is using external light and heat to direct nanorobots to a site, and following up with a way to encourage coordinated activity so the nanorobots take on therapeutic jobs that go beyond delivering drugs.
Self-powered “bots”
In a particularly noteworthy accomplishment, nanoengineers from the University of California, San Diego (UCSD), constructed hybrid microrobots that eliminated pneumonia-causing bacteria from the lungs of mice, leading to recovery of every single mouse [2]. In comparison, all of the mice that received intravenous (IV) antibiotics at the same drug dosage died from the infection within three days. “It is because only a very small fraction of the IV-administered drug really gets into the lungs, but with the microrobots, we had 100% of the drugs entering the lungs and retained in the lungs,” reported Liangfang Zhang, Ph.D., professor in the UCSD Jacobs School of Engineering (Figure 1). The research groups of Zhang and UCSD Professor Joseph Wang, Ph.D., co-developed the microrobots.
Figure 1. Liangfang Zhang, Ph.D., professor in the UCSD Jacobs School of Engineering. (Photo courtesy of UCSD Jacobs School of Engineering.)
The hybrid microrobots are a combination of nanoparticles and algae (Figure 2). Wang’s group screened numerous types of naturally occurring algae to identify a candidate that would remain stable, motile, and alive in lung tissue for an extended period of time. That candidate was a single-celled green microalga (Chlamydomonas reinhardtii) with two long flagella for swift propulsion.
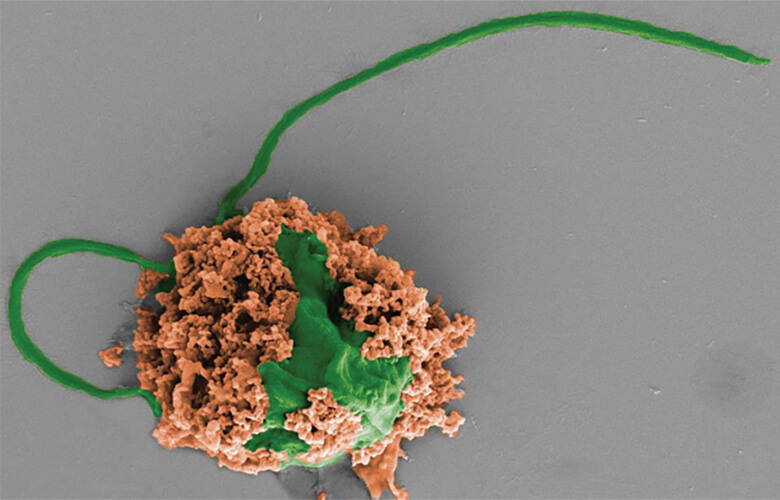
Figure 2. Zhang’s group with that of UCSD Professor Joseph Wang, Ph.D., co-developed a hybrid microrobot made of a living algae cell (green) covered with biodegradable polymer nanoparticles (brown), which can be filled with pneumonia-fighting antibiotics. Tests in lab animals with severe pneumonia showed the hybrid microrobots were highly effective: 100% of lab animals survived infection. (Photo courtesy of Fangyu Zhang and Zhengxing Li.)
Zhang’s group took on the job of designing the nanoparticles using a two-component approach. One component is an antibiotic-filled polymeric core that is surrounded by a neutrophil membrane. The membrane not only stabilizes the nanoparticle, but also neutralizes the inflammatory cytokines that are the host immune system’s response to infection with the pneumonia bacterium. Straightforward click chemistry was enough to attach the nanoparticles to the algal surface. Zhang described, “We modified the neutrophil membrane with one chemical, modified the algal surface with another chemical, and when these two chemicals get close to each other, they just react and link, or ‘click,’ together.”
They tested the microrobots by putting them in an aqueous solution and administering them via a tracheal tube to the lung tissue of pneumonia-infected mice. Once in the lungs, the algae simply started swimming and dispersing. “We tested the distribution in the lungs, and by one hour, we saw the entire lung tissue uniformly covered,” Zhang said. With this direct-delivery approach, the antibiotics were able to release from the polymer core in a sustainable manner and eliminate the pneumonia bacteria.
He further noted that free antibiotic drug could also be administered intratracheally, which would similarly result in all of the drug entering the lung tissue, however free drugs diffuse out of the lungs quickly, whereas the microrobots can retain the drugs in the lungs. In their comparison of the microbot- to the IV-administered drug, he added, “The therapeutic efficacy of the microrobot drug is equivalent to IV-administered free drug that is 3,200 times higher dosage.”
Once the microbots’ job is done, they soon disappear. The biocompatible and biodegradable polymer core metabolizes eventually to water and carbon dioxide, and the body’s macrophages clear the algae, Zhang said. “So, nothing toxic is left behind to cause inflammation or other symptoms.”
Moving forward, the researchers have several objectives for this innovative and early area of research. That includes finding a more benign, patient-friendly administration route. While intratracheal delivery may be an acceptable option for severely ill patients who already have a tracheal tube in place for ventilation, Zhang said, it is not suitable for the broader patient population. The researchers are also looking at other payloads. “For our initial work, we picked one of the most commonly used antibiotics, but we are now considering the possibility of other novel therapeutic drugs, such as RNA-based therapeutics, but how they work with these self-propelled microrobots remains to be studied.”
Cues from nature
Other researchers have also been looking to the natural world, but as design inspiration. One of them is Tian Qiu, Ph.D., biomedical engineer at the University of Stuttgart Institute of Physical Chemistry and research group leader at the large European research consortium called Cyber Valley (Figure 3).
Figure 3. Tian Qiu, Ph.D., biomedical engineer at the University of Stuttgart Institute of Physical Chemistry and research group leader at the large European research consortium called Cyber Valley, holding a microrobot. (Photo courtesy of Sven Cichowicz/University of Stuttgart.)
Formerly, Qiu was a key part of the Max Planck Institute for Intelligent Systems’ research group that in 2018 reported it had not only developed a nanorobot with a tail reminiscent of the helical flagella seen in bacteria, such as Escherichia coli, but that the nanorobot could also traverse dense tissue [3].
For that project, Qiu built a microrobot that was 300 microns in diameter and nearly a millimeter long, he described. “We tested it in simple Newtonian fluids, such as water or silicone oil, and it worked perfectly. When the helical tail rotated clockwise, the right-handed helical robot moved forward; and when it rotated counterclockwise, it moved backward.” They then tried it in tissue, specifically the dense vitreous of the eye, but found that a protein mesh in the tissue trapped the microrobots. They eventually solved the problem by fabricating smaller robots—nanorobots—and adding a slippery coating to allow them to slide through the fine mesh (Figure 4).
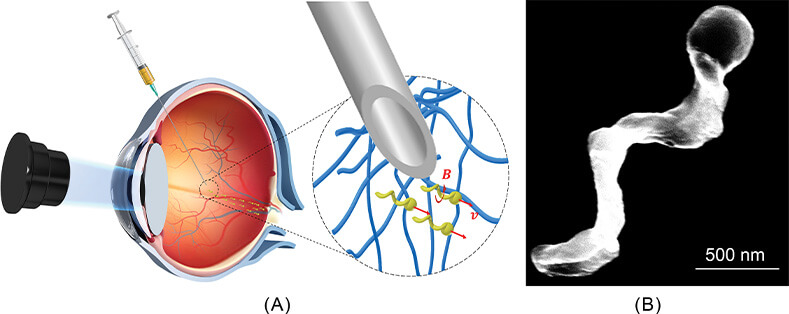
Figure 4. Qiu was part of the Max Planck Institute for Intelligent Systems research group—under the direction of Peer Fischer, Ph.D., who is now at the Max Planck Institute for Medical Research—(a) that developed nanorobots that could penetrate the dense tissue of the eye. The nanorobots had a helical tail that corkscrewed through the vitreous to reach the target site. Rotation in one direction (b) moved the nanorobot forward (v), and rotation in the other direction moved the nanorobot backward. Inset image shows the actual nanorobot. (Images courtesy of Tian Qiu, University of Stuttgart.)
Now with his own research group, Qiu is once again exploring the potential of microrobots with one of the prestigious European Research Council (ERC) Starting Grants, which began in January 2023. “While nanorobots could be used to deliver drugs, they can only carry so much cargo, so what I want to bring with this project, called VIBEBOT, is to add more cargo, such as microelectronics, sensors, and even electrodes, and still pass through soft tissue,” he said. “Because we are at a larger scale—hundreds of microns or the submillimeter scale—we cannot sneak through the mesh and instead will have to determine the best way to break the net.”
He will also be exploring various advanced medical imaging techniques to localize and control the microrobots. Every current imaging technique has its challenges, he said. “Magnetic resonance imaging is probably not the best one for us, because magnetics is already used for actuation. Computed tomography may have the resolution, but it uses radiation and is relatively slow, so you can’t have real-time feedback. X-ray imaging fluoroscopy is possible, but still releases radiation and we don’t want continued, real-time, 30 Hz imaging of the human body,” he related. “We also thought about ultrasound imaging, which is basically harmless and is real-time, but the problem is that the resolution is borderline for microrobots, and just forget about it for nanorobots.”
With the VIBEBOT project, Qiu’s group has also aimed for a new method that can do “real-time magnetic localization of submillimeter devices with submillimeter resolution in very deep tissue, even 10 cm distance.” His group has positive preliminary results and a patent application ready to go, but he cannot go into detail about the method until the associated journal article is published so he can describe it further. He would say, however, that this localization technique “can be tracked in real time, radiation-free, and deep within the body” and added that he and his group are developing the technology as a portable machine that clinicians can roll to a bedside.
Through the microrobots and the new localization technique, Qiu envisions a wide range of biomedical possibilities. “For instance, microrobots could deliver a larger amount of drugs, perhaps as an implant, to a solid tumor,” he said. “Beyond drugs, perhaps we can deliver wireless sensors or stimulators into the soft tissue, perhaps reaching deep into the soft tissue of the brain. There are many, many applications like this.”
Control and coordination
Until technology can further reduce the size of actuators and sensors for micro-/nanoscale on-robot integration so that micro- and nanorobots can navigate by themselves, external control will continue to be a prime research area. One way to provide that control is with heat and light, according to Yuebing Zheng, Ph.D., associate professor of mechanical engineering at the University of Texas, Austin (Figure 5). His group is doing side-by-side development of both the bots and the optothermal method to direct them.

Figure 5. Yuebing Zheng, Ph.D., associate professor of mechanical engineering, and materials science and engineering at the University of Texas, Austin. (Photo courtesy of Yuebing Zheng.)
“At its early stage, our work was inspired by the Nobel Prize-winning technology of optical tweezers, in which laser beams are applied to control biological cells and micro- and nanoparticles,” Zheng said. On the positive side, light has advantages in that it can be freely adjusted, and requires no physical contact with the target, making it possible to direct the robots remotely. On the other hand, the force provided by light is very weak. “Generally, the force can be felt at the micro/nanoscale only if you’re using a high-intensity laser,” he said, “but that would cause damage to those robots made of the kinds of polymer biomolecules necessary for in vivo applications.”
This conundrum led Zheng and his group to consider how they could use low-power light to generate high force. Their answer was thermophoresis. So, rather than using the high-power light to directly move the robots, they would use low-power lasers to produce heat gradients, or slightly heated spots that are then surrounded by cooler areas. “Physicists had already discovered that micro/nanoparticles can move directionally on a temperature gradient, so the idea was to use optical heating to generate that temperature gradient and control the motion of micro- and nanorobots,” he said.
The researchers then put their idea to the test. They combined an ultralow-power laser beam with a simple optical setup; built gradient-sensitive particles of a variety of materials ranging from gold and other metals to polymer ceramics; and added a feedback-control system. With all the parts in place and working together, the researchers demonstrated they could provide programmable, multimodal, and optothermal control of micro/nanoparticles [4], which could, in turn, control micro- and nanorobots (Figure 6).
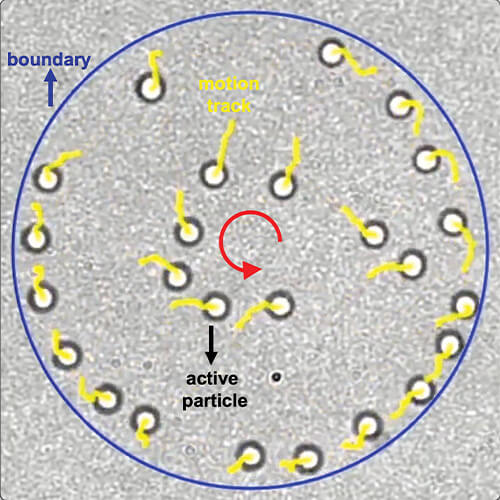
Figure 6. Zheng and his group are using low-power lasers to produce heat gradients, which can be used to control the motion of micro- and nanorobots, as well as feedback systems that allow for their directed, collective motion. Such collective motion is critical for delivering sufficient quantities of drugs and for performing other tasks that the nanorobots could not accomplish individually due to their limited size and power. (Image courtesy of Yuebing Zheng.)
Zheng and his group are now taking the technology one step further and using the same optothermal principles to direct micro- and nanorobots to function collectively, something that computer scientists call swarm intelligence, or physicists and chemists call collective motion. “This is an advantage of light again, because it can not only control them, but can also be used as an imaging tool,” he explained. “So, we have external imaging capability to monitor where each particle is, and we can then use the imaging as feedback to tell our system to adjust the control beam depending on the particles’ relative distance and velocity, and therefore realign them [5]. It’s the same thing that happens with birds in a flock, but birds have eyes to see their neighbor, and can use that information to adjust their position and their speed. The nanoparticles instead rely on the external laser beam and our feedback loop to direct them.”
So far, the group has made “good progress” to demonstrate collective motion in vitro, and is now moving into in vivo applications. “The in vivo imaging of nano/microparticles becomes more challenging: we need to incorporate a sophisticated imaging system into our control platform so we can achieve the same resolution and time response (as our in vitro results), which are required for the feedback control we need.” They also hope to incorporate machine learning to make the data processing and imaging more efficient and accurate.
Because their work is a platform technology, Zheng views it as having broad applications for biomedical treatment, but also in nonbiomedical areas. “You can imagine building micro- and nanorobots that can remove bacteria, viruses, or microplastics from water, or using the feedback loop for driverless cars and autonomous drones that rely on collective motion to achieve better traffic maneuverability or better task performance,” he remarked. “With our platform technology, we hope that these goals can be achieved earlier.”
References
- S. Wilhelm et al., “Analysis of nanoparticle delivery to tumours,” Nature Rev. Mater., vol. 1, no. 5, May 2016, Art. no. 16014.
- F. Zhang et al., “Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia,” Nature Mater., vol. 21, no. 11, pp. 1324—1332, Nov. 2022.
- Z. Wu et al., “A swarm of slippery micropropellers penetrates the vitreous body of the eye,” Sci. Adv., vol. 4, no. 11, Nov. 2018, Art. no. eaat4388.
- H. Ding et al., “Programmable multimodal optothermal manipulation of synthetic particles and biological cells,” ACS Nano, vol. 16, no. 7, pp. 10878—10889, Jul. 2022.
- H. Ding et al., “Universal optothermal micro/nanoscale rotors,” Sci. Adv., vol. 8, no. 24, Jun. 2022, Art. no. eabn8498.