Among the most common sleep disorders are those related to disruptions in airflow (apnea) or reductions in the breath amplitude (hypopnea) with or without obstruction of the upper airway (UA). One of the most important sleep disorders is obstructive sleep apnea (OSA). This sleep-disordered breathing, quantified by the apnea–hypopnea index (AHI), can produce a significant reduction of oxygen saturation and an abnormal elevation of carbon dioxide levels in the blood. Apnea and hypopnea episodes are associated with arousals and sleep fragmentation during the night and compensatory response of the autonomic nervous system.
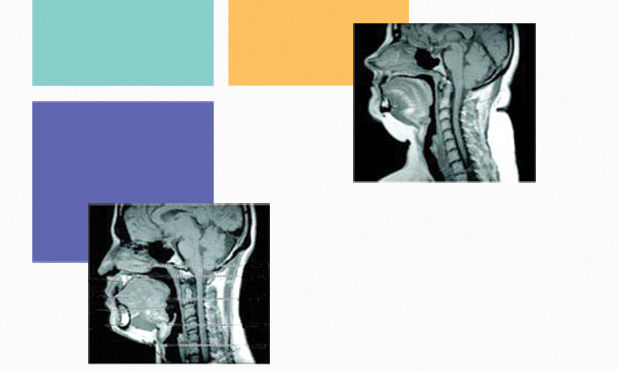
OSA is a serious and common disorder that causes important long-term sequelae, such as hypertension and neurocognitive effects, producing daytime sleepiness. It is also associated with an increased risk of stroke, cardiac arrhythmias, coronary disease, motor vehicle crashes, and other issues. One of the main physiological mechanisms of OSA is the low activity of the UA dilator muscle during sleep, which facilitates the collapse of the pharyngeal airway [1], [2]. The UA size is usually different in severe OSA patients [3] (Figure 1). Although OSA is a highly prevalent medical condition, there are no pharmacological or surgical approaches that can solve most of these cases.
![FIGURE 1: A midsagittal magnetic resonance image (MR I) (a) in a normal subject and (b) in a patient with severe OS A. Highlighted are the four UA regions (nasopharynx, retropalatal region, retroglossal region, and hypopharynx), UA soft tissue (soft palate, tongue, and fat), and craniofacial structures (mandible). Fat deposits are shown in white on the MR I. Note that in the apneic patient: (a) the UA is smaller in both the retropalatal and retroglossal region, (b) the soft palate is longer and the tongue size is larger, and (c) the quantity of subcutaneous fat is greater. (Images from [2, Fig. 3] and [3] and reprinted with permission of the American Thoracic Society. Copyright © 2014 American Thoracic Society.)](https://www.embs.org/wp-content/uploads/2014/09/jane01-2339292.jpg)
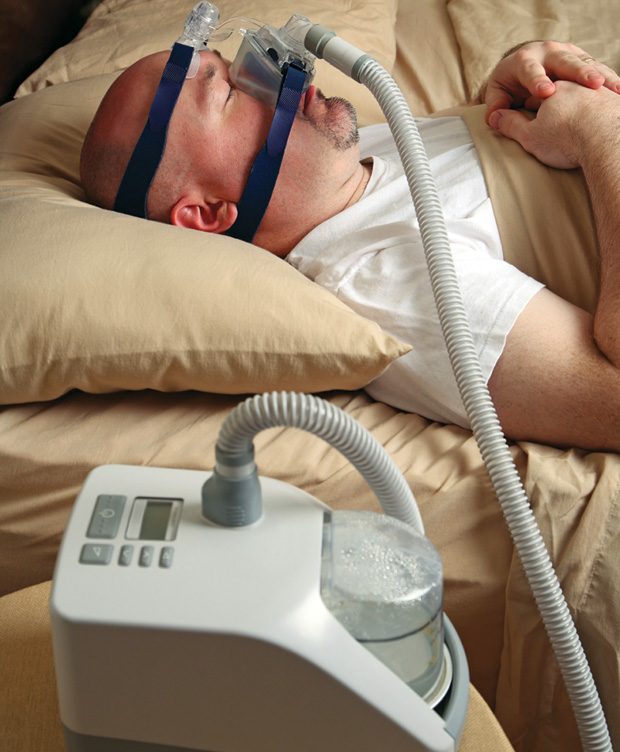
The pioneering work of Sullivan et al. described, in 1981, a new noninvasive therapy based on a device that supplied continuous positive airway pressure (CPAP) to OSA patients during the night. The results were successful and, very soon, the first commercial CPAP devices became the standard therapy for OSA. These devices are based on a flow generator that pushes air through a tube to a face mask (Figure 2). Then, the air passes through the throat, where the positive pressure ensures that the UA remains open, avoiding apnea and hypopnea episodes.
The contribution of CPAP therapy to improving the health and quality of life of OSA patients has been validated in many research and epidemiological studies [4], [5]. The standard CPAP machine delivers a constant positive pressure during sleep, usually ranging from 4 to 20 cm H2O. This means that a constant value (e.g., 10 cm H2O) is applied all night during inspiration, exhalation, and apnea episodes. This constant positive pressure keeps the UA open during the respiratory cycle, but it could be a cause of discomfort for patients. Currently, CPAP therapy is most commonly used as a primary treatment for patients with sleep apnea as it is the most effective option to reduce the AHI, reversing UA obstruction during sleep and improving sleep structure.
Nevertheless, the treatment effectiveness of CPAP therapy is limited by a very poor adherence by patients, with the minimum use defined as four hours per night. It has been reported that 46–83% of patients with OSA were nonadherent to classical CPAP therapies [6]. However, recent advances in CPAP systems have slightly improved those results.
Therefore, one of the major challenges in OSA therapies is the improvement of patients’ adherence to CPAP devices [4]. Among the main factors that could increase the use of CPAP devices and predict the lack of adherence are: issues in mask design (oral and nasal masks, and nasal pillows), patient characteristics, disease severity, side effects, method of CPAP initiation, claustrophobia, and physiological factors.
In the last few years, many interventions have been proposed to overcome the limitations of classical CPAP devices, including humidification of the airway, better fitting of CPAP parameters according to the severity of the disease and individual patient characteristics, and, more recently, adding active behavioral and psychological interventions with patients. Moreover, in contrast to pharmacological treatments that use standard doses for patients, CPAP therapy requires an adjustment personalized for each patient, and the optimal parameters could vary substantially throughout the treatment or even during the night.
Consequently, many biomedical engineering efforts have been devoted to improving the design of CPAP machines. In particular, the positive airway pressure (PAP) has been adjusted based on the severity of the condition of OSA patients, avoiding a constant pressure value of CPAP therapy and including different control algorithms. The main challenge is to enhance the comfort of patients, increase the adherence to the therapy, and to be more effective.
New Intelligent Adaptive PAP Devices for OSA therapy
One key issue in the design of new therapeutic devices has been the management of a PAP variable according to respiratory patterns on a breath-by-breath basis [6], [7]. In this way, different so-called PAP modalities have been proposed.
The operating principle of automatic PAP (APAP) machines is similar to classical CPAP machines, which use a single constant value of PAP, but, in this case, the pressure is adapted for the night according to the needs of patient and his or her airway obstruction. Among others, sleep stages, position, and inspiratory flow limitation can suggest different pressure-level settings during the night or between nights. A study of the potential advantages of APAP over CPAP [8] concluded that APAP produced a significant reduction in AHI compared to CPAP, but, at the same time, allowed lower levels of PAP pressure to be applied (Figure 3).
![FIGURE 3: The mean (in blue) and maximum (in yellow) pressure with the various CPAP devices. The data are presented as mean +/- SD. *p < 0.05 versus fixed CPAP; #p < 0.05 versus AutoSet; ¶p < 0.05 versus Horizon; +p < 0.05 versus Virtuoso. (Figure used with permission from [8].)](https://www.embs.org/wp-content/uploads/2014/09/jane03-2339292.jpg)
Various APAP equipment uses different complex algorithms, which, in many cases, are proprietary or patented by different commercial manufacturers. These devices are known also as auto-adjusting PAP, auto-titrating PAP, self-adjusting PAP, or simply auto PAP. Usually, they adjust the pressure values only after a respiratory event related to OSA occurs, such as apnea, hypopnea, or snoring episodes. More sophisticated APAP devices consider respiratory flow limitation, analyzing the flatness and shape of respiratory flow to predict an obstructive event of UA and adjusting the pressure parameters. The differences between APAP devices were studied by the American Academy of Sleep Medicine in 2008 [9], and it was stated that only certain APAP equipment is appropriate for titration and long-term OSA therapy.
Another approach to PAP therapy is the so-called Bi-level PAP. This device uses two fixed levels of positive pressure unlike the classical CPAP machine, which uses a constant value all night. One higher level corresponds to the inspiratory pressure (IPAP), and a lower pressure (EPAP) is applied during the expiratory phase. This strategy prevents the imposition of unnecessarily high pressure during expiration. For instance, the device can supply an IPAP of 15 cm H2O, while the EPAP is adjusted to 11 cm H2O. Many commercial BiPAP devices use their trade names and, therefore, are more widely known as BiPAP orVPAP. These PAP devices are usually recommended when a high CPAP pressure (e.g., 15 cm H2O) is prescribed, and it could be beneficial to apply a lower EPAP or, in general, to increase comfort during exhalation.
Some manufacturers offer an advanced version of Bi-level PAP that includes S/T function. Bi-level S/T devices can operate in spontaneous (S) mode, as a traditional Bi-level, or in timed (T) mode, when the device controls inhalation and exhalation time independently of the spontaneous breathing of the patient. In fact, this device in T mode acts as a noninvasive ventilator that manages the time for the respiratory cycle. Patients with neuromuscular disease, chronic obstructive pulmonary disease, or obesity hypoventilation are the best candidates for this therapy.
Technical combinations of both APAP and Bi-level PAP have generated a new type of device: auto Bi-level systems. In these systems, the IPAP and EPAP settings can be adapted during the night according to respiratory episodes and the requirements of the therapy. This permits a better resolution of apnea, hypopnea, flow limitation, and snoring events. Usually, these devices use 3–8 cm H2O of difference between the current values of IPAP and EPAP.
On the other hand, improved algorithms for APAP have been proposed using a pressure relief concept to design a more flexible PAP [6]. The objective is to match the pressure delivery to the patient with a better adjustment to the breath-by-breath profile. This allows for a reduction of positive pressure at the beginning of exhalation according to the individual respiratory flow while tracking the patient’s breathing during sleep. Two examples of these relief technologies are the patented digital autotrack and sensitivity algorithms included in the C-Flex and A-Flex devices (Philips Respironics, United States) or Expiratory Pressure Relief (ResMed, Australia). Recent studies show that C-Flex and A-Flex devices (Figure 4) improved the respiratory indices of OSA and sleepiness with better comfort and satisfaction for patients, thereby increasing adherence [7], [10].
![FIGURE 4: (a) C-Flex pressure relief reduces pressure at the beginning of exhalation and returns to the therapeutic pressure just before inhalation. The level of pressure relief varies based on the patient’s expiratory flow and based on which of the three C-Flex settings (1, 2, or 3) has been selected. (b)–(d) A-Flex has the same function as C-Flex during expiration. (b) At 4 cm H2O CP AP, the A-Flex PAP machine is same as the C-Flex PAP machine. (c) At 5 cm H2O CP AP, the A-Flex PAP machine can provide 1 cm H2O pressure support at inspiration. (d) From 6 or more cm H2O CP AP, the A-Flex machine can provide 2 cm H2O pressure support at inspiration. (Figure used with permission from [10].)](https://www.embs.org/wp-content/uploads/2014/09/jane04-2339292.jpg)
A different approach is the design of PAP devices oriented to specific pathologies, such as central sleep apnea (CSA) and related respiratory patterns. The coexistence of CSA and OSA is very common in patients with heart disease. Adaptive servoventilators (ASVs) are the PAP devices appropriated to treat these complex sleep apneas that combine OSA, CSA, periodic breathing, and Cheyne–Stokes respiration (CSR). An ASV device usually combines its function as a ventilator and Bi-level PAP, suppressing CSA/CSR and OSA.
Future Trends to Improve PAP Therapy and Patient Adherence
The advances in PAP therapy have focused on improvements of pressure supply algorithms to OSA patients. Intelligent adaptive positive pressure and tracking of respiratory flow signals to adjust to the requirements of patients have been the major relevant contributions of recent PAP devices. Currently, different manufacturers offer a wide range of PAP equipment that permit personalized therapy to the patient in an autoadaptive way. Furthermore, the cost-effectiveness of CPAP therapy for OSA patients has been recently confirmed.
Nevertheless, the issue of patient adherence to PAP therapy is still a huge challenge. To solve this problem, the current trends in new PAP systems are oriented toward having the patient participate actively in the management of his/her therapeutic process using the new tools of information and communication technology. Recently, new mobile applications and Web sites designed to engage OSA patients seem to be the key to improving adherence and achieving a more efficient therapy. Therefore, a new generation of intelligent PAP devices, in a framework of m-Health and Tele-Health, seems to be the tool that will permit improved therapy with better participation and motivation of patients.
References
- S. Jordan, D. G. McSharry, and A. Malhotra, “Adult obstructive sleep apnoea,” The Lancet, vol. 383, no. 9918, pp 736–747, 2014.
- J. A. Dempsey, S. C. Veasey, B. J. Morgan, and C. P. O’Donnell, “Pathophysiology of sleep apnea,” Physiol Rev., vol. 90, no. 1, pp. 47–112, Jan. 2010.
- R. J. Schwab, K. B. Gupta, W. B. Gefter, L. J. Metzger, E. A. Hoffman, and A. I. Pack, “Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls,” Am. J. Respir. Crit. Care Med., vol. 152, pp. 1673–1689, 1995.
- P. Gay, T. Weaver, D. Loube, and C. Iber, “Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults,” Sleep, vol. 29, no. 3, pp. 381–401, 2006.
- E. Terri, C. Mancini, G. Maislin, J. Cater, B. Staley, J. R. Landis, K. A. Ferguson, C. F. P. George, D. A. Schulman, H. Greenberg, D. M. Rapoport, J. A. Walsleben, T. Lee-Chiong, I. Gurubhagavatula, and S. T. Kuna, “Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea. Results of the CPAP apnea trial North American program (CATNAP) randomized clinical trial,” Am. J. Respir. Crit. Care Med., vol. 186, no. 7, pp. 677–683, 2012.
- T. E. Weaver and R. R. Grunstein, “Adherence to continuous positive airway pressure therapy,” in Proc. American Thoracic Society, vol. 5, no. 2, 2008, pp. 173–178.
- J. F. Garvey and W. T. McNicholas, “Continous positive airway pressure therapy: New generations,” Indian J. Med. Res., vol. 131, no. 2, pp. 259–266, 2010.
- A. Stammnitz, A. Jerrentrup, T. Penzel, J. H. Peter, C. Vogelmeier, and H. F. Becker, “Automatic CPAP titration with different self-setting devices in patients with obstructive sleep apnoea,” Eur. Respir. J., vol. 24, pp. 273–278, 2004.
- T. I. Morgenthaler, R. N. Aurora, T. Brown, R. Zak, C. Alessi, B. Boehlecke, A. L. Chesson, Jr., L. Friedman, V. Kapur, R. Maganti, J. Owens, J. Pancer, and T. J. Swick, “Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: An update for 2007. American Academy of Sleep Medicine report,” Sleep, vol. 31, no. 1, pp. 141–147, 2008.
- Y. Chihara, T. Tsuboi, T. Hitomi, M. Azuma, K. Murase, Y. Toyama, Y. Harada, K. Aihara, K. Tanizawa, T. Handa, C. Yoshimura, T. Oga, K. Yamamoto, M. Mishima, and K. Chin, “Flexible positive airway pressure improves treatment adherence compared with auto-adjusting PAP,” Sleep, vol. 36, no. 2, pp. 229–236, 2013.