It seems simple: send a small electrical current to a major nerve in the body and stimulate hormones and organs to react in the way you want. New efforts by research teams are doing just that, zapping peripheral nerves attached to major organs in the hopes of addressing problems as diverse as inflammatory bowel disease, chronic pain, and posttraumatic stress disorder. Thanks to the continued advance of smaller and more efficient electronics, researchers are finding new ways to develop implantable bioelectrical devices to treat a wide range of ailments.
Techniques harnessing electricity to treat afflictions have been around for decades. Electroconvulsive therapy can alleviate depression, for example, while pacemakers use electrical currents to regulate heartbeats. More recently, a technique called deep brain stimulation—essentially a pacemaker for the brain—has had success in Parkinson’s disease and epilepsy patients whose symptoms cannot be controlled by other treatments. (FDA-approved devices are already on the market for these uses.) Now, a Defense Advanced Research Projects Agency (DARPA) program called the Electrical Prescriptions (ElectRx) project is underway, recently announcing funding to seven teams spread across the United States and one in Australia to explore the potential of minimally invasive bioelectrical devices that act on peripheral nerves and glands to affect organs in the body—including the brain (Figure 1).
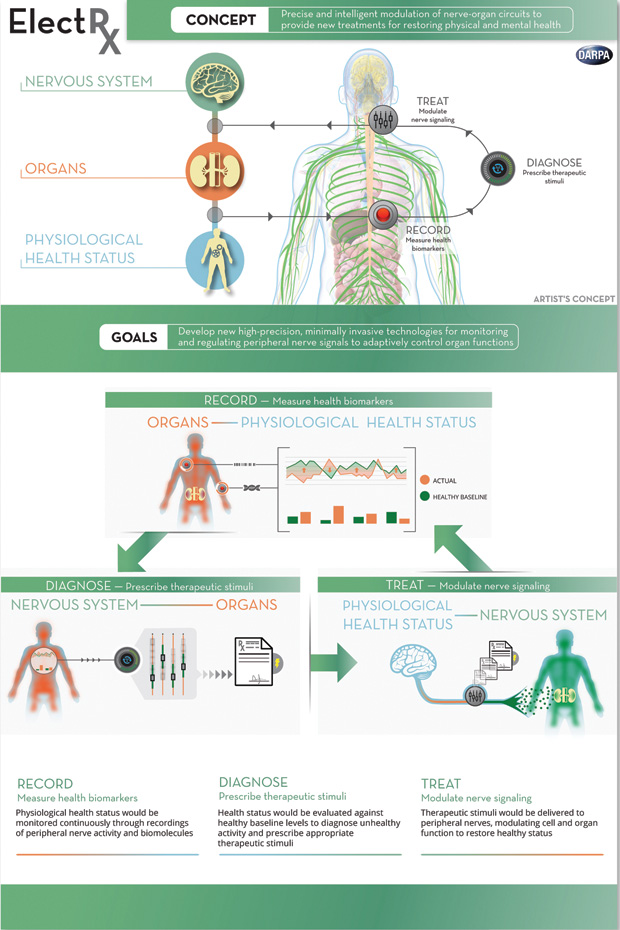
“The peripheral nervous system is a gateway to almost every aspect of human physiology,” says Doug Weber, biomedical engineer and manager of the ElectRx program. The applications of establishing direct communication with nerves could eventually include more advanced prosthetic limbs, drug-free regulation of blood pressure, restored muscle control in cases of paralysis, and improved stress and allergy management, to name a few. Weber adds, “Our goal is to leverage innate functions of the nervous system to monitor and treat disease without drugs or surgery. The clinical implications are profound.”
Resetting the Mind
Robert Rennaker, department chair for Bioengineering at the University of Texas (UT) in Dallas, knows firsthand the effects of war. After spending five years in the Marine Corps, he now dedicates his research efforts to addressing conditions that afflict soldiers exposed to explosives, including phantom limb pain and posttraumatic stress disorder (PTSD) as well as chronic pain and tinnitus—many of which have no good treatments. “Someone exposed to an improvised explosive device—IED—has an average of six neurological conditions,” Rennaker notes.
When an explosion detonates, the accompanying sensory input—a billow of smoke, a black SUV—can become etched into a person’s memory and be permanently associated with the fear response. This is essentially a survival mechanism: the next time the person sees, say, a similar SUV or funnel of smoke, the body prepares a preemptive fight-or-flight response to help him or her escape faster. However, this survival response becomes posttraumatic stress disorder when the sight of every SUV on a suburban road, for example, still sets off that fight-or-flight response. “Neural circuits involved in sensory [input] and in fear have become connected. Now you have a person who has a fear response they can’t control,” explains Rennaker.
The device Rennaker uses (Figure 2), like many electrostimulation efforts outside the brain, focuses on the vagus nerve, the body’s longest cranial nerve. It begins in the brain, runs through the neck, and splits into many branches and fibers to connect with nearly all the major organs. Rennaker’s team has spent the past several years testing how electrical stimulation of the vagus nerve, paired with sensory or motor output, can drive reorganization of the nervous system.

Sending an electric pulse to the vagus nerve elicits a host of reactions; most notably, it starts a cascade of events that leads to the release of neurotransmitters. Through vagus nerve stimulation, Rennaker believes he can rewire the nervous system by manipulating neuroplasticity, the ability of neurons to make connections and—essentially—learn anew. “It’s analogous to rewiring a circuit board,” he says. By timing this release of neurotransmitters to sensory stimuli—for example, the sight of an object—with the sensation of being calm, Rennaker hopes to reorganize the brain’s circuitry when it goes awry in PTSD.
To do this, Rennaker shows subjects the stimulus that triggers their condition—such as the SUV—and coaxes them into a relaxed state through exercises or repetitive viewing of the stimulus (or something similar to it) in a safe setting. When the subject feels calm, Rennaker uses a small device to stimulate the vagus nerve. This stimulation seems to strengthen any neural connection or activity that is active at the moment and accelerates the learning experience, according to Rennaker.
By repeatedly stimulating subjects’ vagus nerves while they are feeling safe and viewing the trigger for their anxiety, Rennaker is trying to reteach the brain to associate a feeling of being relaxed with the stimulus, negating the fear reaction that has previously resulted in symptoms of PTSD. Through the DARPA program, Rennaker hopes to refine and optimize this procedure, moving it from the research laboratory to the clinic.
This same method, Rennaker says, also works with rehabilitation after a stroke. The team has carried out three clinical trials (and 20 trials in animal models) focusing on motor deficits, spinal cord injury, and stroke that have shown safety and some efficiency. By pairing a specific movement to the stimulation of the vagus nerve, his team can enhance or reorganize the brain to drive specific movements. For example, when the team stimulates the vagus nerve as a subject moves his or her shoulder, the area of the brain involved with shoulder movement lights up and makes the shoulder movement easier in the future. In a six-week clinical trial, stroke patients who received vagus nerve stimulation along with traditional rehabilitation reported greater improvement in movement than those who had only the traditional rehabilitation [1].
The UT Dallas team also uses the technique to alleviate tinnitus, a constant internal ringing or noise that has few treatments and can be so debilitating it drives some patients—in extreme cases—to suicide. By stimulating the vagus nerve in 40 patients while playing tones above and below the frequency of the tinnitus tone, the team succeeded in reducing symptoms in about half of participants in an ongoing study [2]. The technique has already proven successful in animal models, according to research reported in 2011 in the journal Nature [3]. The researchers are also investigating how to enhance learning through this technique, particularly for people with cognitive disorders.
Disorders like PTSD and tinnitus take a huge toll on soldiers, says Rennaker, and traditional treatments can cost hundreds of thousands of dollars. But he and his team hope to change this: “We think with this DARPA initiative we can get the surgery and device down from US$30,000 to US$2,000.” Right now, the team uses a FDA-approved vagus nerve stimulator about the size of a silver dollar that is implanted into the chest with a lead reaching up to part of the vagus nerve in the neck. The stimulator goes on and off for about half a second when triggered by a therapist.
The researchers are developing their own device, about the size of a pill, that could be implanted into the neck in a 45-minute outpatient surgery. The device doesn’t have a battery but would require a patient to wear a necklace with a radio-frequency (RF) coil that would remotely power the device (Figure 3).

“We’re going to take traditional therapies and pair them with our device to help everyone recover faster,” Rennaker says. “Eventually we envision this as a platform technology open for others to develop applications for additional disorders.” Although much is still not known about the brain and mechanisms associated with vagus nerve stimulation, Rennaker likens electrostimulation to immune system therapies: before scientists knew precisely how the immune system worked, they were still able to use the plasticity of the acquired immune system to develop vaccines. “We found out how to drive plasticity in the nervous system. We haven’t mastered it completely but understand it well enough to potentially treat a wide range of neurological symptoms.” Still, Rennaker admits he gets some pushback from the medical community: “People say you can’t use neurological plasticity to treat a neurological condition. But we’re seeing that you can.”
Controlling Immunity
Another target for electrical stimulation is the immune system. A team in the ElectRx program at Purdue University is exploring what happens to the immune response—and inflammation in particular—when the vagus nerve is stimulated. The Purdue team—led by Pedro Irazoqui, professor at the schools of Biomedical Engineering and Electrical and Computer Engineering as well as director of the Center for Implantable Devices, and in collaboration with London-based LivaNova—is exploring which branch of the vagus nerve below the diaphragm results in an immune system response.
As previous research has shown, one or more branches innervate the small intestine and, when stimulated, may suppress the release of inflammatory proteins called cytokines. Over the past few years, the medical community has explored inflammation and the overabundance of cytokines as being a potential cause for depression. “Right now there isn’t a consensus on the mechanisms on how the nerve drives a reflex to stimulate the immune response,” says Irazoqui. “Several teams are exploring that as well as the optimal way to modulate the release of cytokines to relieve conditions like depression and PTSD.”
To study this and other medical applications for stimulating different branches of the vagus nerve, the team has developed the Bionode (Figure 4), a wireless, implantable medical device that harvests electromagnetic energy to communicate with a phone or computer. By getting rid of the battery and wirelessly transferring power efficiently, Irazoqui believes they will be able to make a consumer device ten times smaller than what is currently on the market as well as something that wouldn’t have to be replaced through a second surgery. Currently, most equivalent medical devices are about the size of a watch and contain a lithium battery.
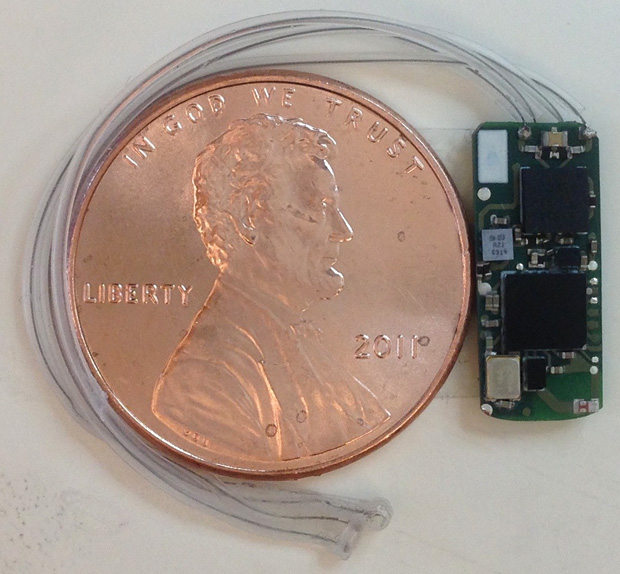
Aside from being wirelessly powered, the Bionode will also customize how it stimulates the nerve. “Virtually every medical device that stimulates the nerves uses a constant current or voltage,” Irazoqui says. However, these become less effective over time because the body grows desensitized. The Bionode, by contrast, will record the body’s response and adapt and modify its current continuously to give consistent treatment, according to Irazoqui. His team is testing the Bionode in animals now and plans to test it in people in year four of the DARPA program.
“The technology has improved dramatically. We can design our own custom circuits and compress a lot of the electronics in a tiny sophisticated package, allowing us to interface the nervous system and electronics seamlessly to deliver these electrical currents,” Irazoqui says. “Comparing something like the Bionode to a pacemaker is like comparing a modern laptop to a punch card computer in the 50s.”
Outside of the DARPA project, Irazoqui’s team has also been working on “electroceutical” therapy for controlling the bladder. The researchers developed a device that can record bladder pressure and stimulate the pelvic nerve to prevent involuntary release of urine—a problem for many people over 60.
Soothing the Gut
Stimulating the vagus nerve, it turns out, can even help ease common but hard-to-treat gastrointestinal ailments, which two teams in the DARPA project are exploring. “Inflammatory bowel disease (IBD) is a common and debilitating condition for which there is no adequate treatment,” says John Furness, director of the Florey Institute’s Digestive Physiology and Nutrition Laboratories in Melbourne, Australia. His team is working with the Bionics Institute, also in Melbourne, and other collaborators to develop an implant that would stimulate the vagus nerve in a way to induce an anti-inflammatory effect in the digestive tract.
Jiande Chen, professor and director of the Gastrointestinal Motility Laboratory at Johns Hopkins University, likewise aims to treat IBD, but by targeting the sacral nerve. Sacral nerve stimulation is thought to cause the secretion of acetylcholine, resulting in the suppression of inflammatory-causing cytokines.
Both teams are looking for specific proteins (called biomarkers) that signify a change in the level of gut inflammation. Their devices would use the biomarkers to assess the gut’s state and determine the level of the vagus or sacral nerve stimulation needed to reduce inflammation. This, in effect, would create a closed-loop feedback system that could improve ailments automatically, tailoring the electrical stimulation to each individual.
In other related work, Chen’s group uses electrotherapeutic devices to control the gut’s compulsive contractions. “With electric pulses, we can do what’s called stomach pacing—similar to cardiac pacing—and increase propulsive forces in the gut to improve digestion and treat diseases of the stomach,” says Chen. This technique could be used for patients suffering from a lack of movement in the small intestine after related surgeries or even from everyday constipation.
Chen can also do the opposite: pacing the stomach backward so patients suffering from obesity tied to eating too much would have reduced or suppressed stomach contractions and digest slower. The challenge for this application is to develop a device strong enough to stimulate a muscle (as opposed to a nerve) without inducing bloating. If successful, it could potentially even treat diabetes. “These diseases are very common but are very difficult to treat,” Chen notes. “There are medications but they can only be used to treat the symptoms and can’t cure the inflammation.”
Harnessing Heat
Polina Anikeeva, associate professor at MIT’s Department of Materials Science and Engineering, takes a different approach than others in the ElectRx program. Anikeeva creates magnetic nanoparticles that can stimulate the release of hormones in the adrenal glands—organs responsible for the fight-or-flight response. “Our project is a walk on the wild side, even for DARPA,” she says. The adrenal glands, which are responsible for the stress hormone cortisol and the fight-or-flight messenger epinephrine, are central to the broad experience of stress, according to Alik Widge, a psychiatrist-engineer at Massachusetts General Hospital and partner in this project. “What’s very cool about the ElectRx initiative is that it recognizes that the peripheral nerves and organs are really gateways into the brain and that this has been a very underexplored area despite a desperate need for psychiatric treatments,” Widge explains. As it turns out, the release of adrenal hormones is disrupted in depression, PTSD, and other anxiety disorders.
“By being able to modulate or inhibit the adrenal glands at the right time, we hope to make the stress response more adaptive,” says Widge. “For instance, there’s evidence in animal models that having higher epinephrine in your bloodstream right before you learn a new safety memory improves the learning. Having this technology might make us more able to teach those safety memories to patients with anxiety and trauma disorders.”
The adrenal glands are somewhat like an onion, made up of layers of tissue responsible for different hormones. The team uses alternating magnetic fields that trigger the injected nanoparticles— which are about 20 nanometers in diameter—to dispense heat near the glands. This activates heat-sensitive proteins on the tissue’s membranes and causes the release of hormones. The process takes just milliseconds to induce a reaction. By setting off the magnetic nanoparticles in a controlled way, the team hopes to wield the heat response to alleviate disorders. “It turns out that if we are able to deliver local heat to these cells in the adrenal gland, we can trigger the release of hormones,” Anikeeva explains. “The exciting part is that this method is completely wireless and essentially noninvasive.”
Wielding Sound and Light
Another DARPA ElectRx team is using a different type of wave to trigger nerves: ultrasound. “We think targeted ultrasound could be a good option for managing conditions such as chronic pain and neuropathy,” says Elisa Konofagou, professor of Biomedical Engineering and Radiology at Columbia University. Ultrasound— sound waves at frequencies higher than we can hear—has been used medically for decades, perhaps most commonly to develop sonograms. Konofagou’s team is developing wearable devices to stimulate not the vagus nerve but the saphenous nerve.
This nerve runs along the middle of the thigh and is responsible for skin sensation; it is also associated with neuropathic pain as well as back and lower limb pain. By using ultrasound to affect neurons, the researchers hope to modulate pain noninvasively and with minimal side effects. “We know that, as ultrasound propagates through biological tissue, it exerts mechanical pressure on that tissue, which stimulates specific mechanosensitive channels in neurons and causes them to ‘turn on,’” says Konofagou. “So we think that this is a way we can use ultrasound to turn specific nerves ‘on’ or ‘off’ depending on what the treatment calls for.”
Menlo Park’s Circuit Therapeutics, another member of the DARPA initiative, uses light to develop new treatments for chronic pain. In optogenetics, light-sensitive proteins are added to a cell of interest, such as a neuron, and used to activate or inhibit that cell. Although this technique has been used in research efforts to study the brain, Circuit Therapeutics aims to adapt it into a therapy and use optogenetics rather than electric currents to deactivate nerves that might be causing pain. The company states that their approach would minimize the side effects seen from traditional neurostimulation techniques where electrical currents might affect surrounding tissue in addition to the target nerve.
Aside from the DARPA project, a number of other groups are working on using electricity and other types of energy to induce healing. Some aim to be truly noninvasive by targeting nerves from outside the skin in a technique called transcranial direct current stimulation. Enthusiasm for this method extends beyond the laboratory: a host of do-it-yourself forums and groups aim to use off-the-shelf components to build patches or caps that send an electric charge into the neck or scalp in the hopes of stimulating nerves to activate connections that will relieve ailments or improve cognition. So far, however, there is little evidence that these outside-the-body techniques work, particularly for vagus stimulation where higher voltage would be needed to penetrate the neck muscles.
Nevertheless, many research efforts for neurostimulation look promising. Once researchers address a number of challenges, including decoding the physiology of the nerves themselves, improving technical performance of their devices, and satisfying regulatory hurdles, then safe and minimally invasive neurostimulation technologies may become a widespread reality.
References
- J. Dawson, D. Pierce, A. Dixit, T. J. Kimberley, M. Robertson, B. Tarver, O. Hilmi, J. McLean, K. Forbes, M. P. Kilgard, R. L. Rennaker, S. C. Cramer, M. Walters, and N. Engineer, “Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke,” Stroke, vol. 47, no. 1, pp. 143–150, Dec. 2016.
- MicroTransponder Inc. (2016, May 22). Vagus nerve stimulation (VNS) paired with tones for tinnitus. ClinicalTrials.gov identifier NCT01962558. [Online].
- N. D. Engineer, J. R. Riley, J. D. Seale, W. A. Vrana, J. A. Shetake, S. P. Sudanagunta, M. S. Borland, and M. P. Kilgard, “Reversing pathological neural activity using targeted plasticity,” Nature, vol. 470, pp. 101–104, Feb. 2011.