Some babies are born with a rare condition known as esophageal atresia, in which part of the connection between the throat and stomach is missing or nonfunctional. While this was once untreatable and fatal, in recent years surgeons have developed a method using traction to stretch the tissues out on each end until, over time, they are long enough to be sewn together and so substitute for the missing portion of the esophagus. The procedure has allowed many infant patients to go home with a full, normal life ahead of them. But the procedure, which costs US$500,000 and upwards, requires that the patient be fully sedated and unconscious in an intensive-care unit (ICU) for between one and two months to avoid any bodily motions that could tear out internal sutures.
Now, researchers including Pierre Dupont of the Harvard Medical School and Boston Children’s Hospital are working on a promising alternative that might allow these young patients to completely avoid the lengthy sedation period, along with all its risks and expense. The technique (Figure 1), which has so far only been tested on pigs, uses a small implantable robotic system (Figure 2) that pulls on both of the separated esophageal ends but also senses the response of the tissues and can be adjusted correspondingly. This ongoing built-in monitoring and control, along with an improved design that involves no sutures passing out of the body and also provides more uniform tension, could eliminate the need for repeated surgeries and constant X-rays to track progress. And the database that could be built up showing the exact amount of force applied over time and the resulting outcomes might ultimately lead to improvements in the treatment process.
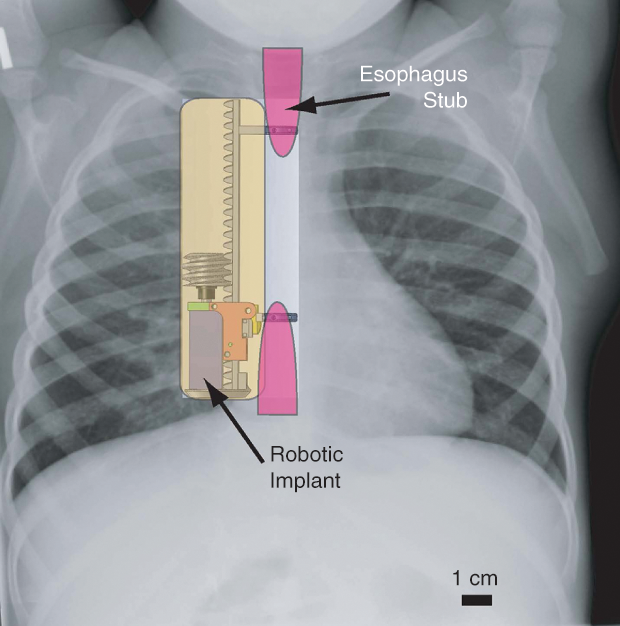
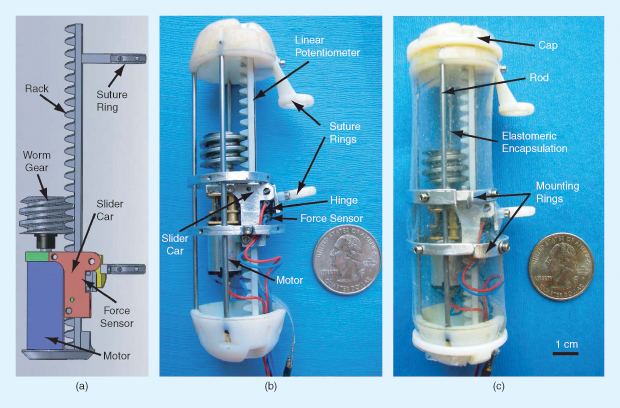
Such research on internal robotics is just one of a wide variety of new approaches being used to harness the latest technologies in service of young patients. The number of such patients may be small compared to the number with major diseases drawing most research funding; but, with their entire lifetimes still lying ahead of them, these young patients’ prospects for a normal life—or even survival—may hinge on bioengineers finding the right new insight and the means to put t Date of publication: 14 July 2017 hat insight into action.
And Dupont’s innovative traction technology is not limited to this particular condition, which affects about 1,000 children in the United States each year. Essentially the same process, with adaptations in the dimensions of the robotic hardware, could be applied to a number of other conditions that also require a slow, steady stretching of tissues, including short-bowel syndrome (Figure 3), which similarly affects about 1,000 children in the United States every year, or circulatory system conditions.

“The goal is to provide a computer-controlled way of applying these traction forces,” Dupont says, with a system that opens up a whole new avenue of potential medical robotics: fully implantable devices that, ultimately, could function with no outside physical connections at all—although the initial prototypes Dupont has been working on at Boston Children’s Hospital still use a small wire for power and data transmission.
Heart Valves That Grow
One of the special challenges in developing biomedical devices for still-growing patients is that anything that gets implanted must dissolve away, be removable in a later surgery, or have the ability to “grow” in some way along with the patient’s own tissues. Pedro del Nido, a surgeon who also works at Boston Children’s Hospital, has been developing an implantable heart valve for young children that does just that: it expands over time, as the child grows.
In fact, several approaches to that problem are being explored, including a valve implant made of a material that chemically changes over time, causing it to expand. Another system uses an implant with embedded tubes that can, over time, be pumped up to expand the whole valve surround using a syringe injected into a small valve on the implant.
Yet another approach, in development by Boston Children’s Hospital’s Juan Melero-Martin, is to implant a kind of scaffolding embedded with stem cells derived from the patient’s own cells and imprinted with its own rudimentary vascular system, which allows the body to essentially grow its own replacement valve in place. Then, because it consists of the patient’s own tissue, the valve will continue to grow with the body, while the scaffolding material simply gets reabsorbed.

Another team working with tissue engineering to address pediatric heart defects is led by Jeffrey Jacot (Figure 4), an associate professor of bioengineering at the University of Colorado’s Anschutz Medical Campus. He has been combining genetic medicine techniques with tissue engineering, also with the goal of making cardiac valve implants that will integrate with the body’s own tissue—in this case, Jacot explains, in the form of patches, conduits, and baffles. “They’re really just ways of reshaping the blood flow and the structure of the heart,” he says.
He starts by harvesting cells from the amniotic fluid, so they are perfectly matched to the particular child. Then, those cells are integrated into a carefully constructed, multilayered scaffold that can be stretched out over time, its layers sliding along each other, to accommodate growth—even as the body’s cells continue to grow to populate that expanding scaffold, which ultimately degrades away.
The implants are currently going through small-animal preclinical testing, Jacot continues. Beyond congenital heart defects (which, he says, affect around 10,000 U.S. children each year), “we’re talking to surgeons about other possible uses” of the same approach, for other kinds of congenital defects. For many such conditions, Jacot continues, “if you don’t end up with a living patch that can grow, you’re going to have lifelong problems.”
Yet pediatric tissue engineering remains an often-overlooked area, Jacot says. “I think the reason is because of the smaller number of patients compared to adults. But when you look at the number of years of life you can impact…,” he trails off meaningfully.
Jacot goes on to note that other groups have also been making strides in the use of stem cells for pediatric medicine, including Christian Breuer, codirector of the tissue engineering program at Nationwide Children’s Hospital in Columbus, Ohio, who is currently doing perhaps the most advanced clinical trials in this area using pluripotent stem cells—the most versatile kind, with the capacity to develop into any type of cell in the body.
Custom Solutions Fit to Measure

Robert Guldberg, director of the Institute for Bioengineering and Bioscience at the Georgia Institute of Technology (Figure 5), says one of the challenges with pediatrics is the years of follow-up required to really assess the long-term outcomes—so important with patients who still have such long potential lifespans ahead.
He cites an example of a child who had a severe congenital condition that leads to a collapsed windpipe. “For these kids, there aren’t really any options; many of them will die,” Guldberg says. But a team of bioengineers at the University of Michigan, including Scott Hollister, developed a polymer-based splint (made of polycaprolactone) that would degrade over a period of years yet keep the airway open throughout that time. But what then?
Guldberg says, “No one knew, once the original material was gone, would it recollapse? But that didn’t happen, and the material is long gone.” However, it took years to fully establish that happy outcome. Such solutions need to be completely customized to the individual patient, he explains. In that particular case, the airway splint was three-dimensionally printed to fit perfectly.
Guldberg says many useful projects have come from a longstanding collaboration between Georgia Tech, which has the nation’s largest biomedical engineering program, and the Children’s Hospital of Atlanta, which has the nation’s largest caseload of pediatric patients.
One of these, according to Guldberg, is “a system that integrates sensors into medical devices, [which] would provide an early warning system if the device was failing.” For example, with spinal implants to treat scoliosis or other deformations, he says, after surgery “typically we use X-rays to verify that fusion has occurred. An implant device with sensors could potentially avoid the need for that.”
Bringing Big Data to the Smallest Patients

Another growing area of pediatric biomedical research is in the collection, interpretation, and use of big data sets. Making use of the large patient base in the Atlanta system, researchers including May Wang, a professor of biomedical engineering and a Kavli Fellow at Georgia Tech (Figure 6, right), have been applying the latest biomedical informatics approaches to pediatric medicine. Their work spans the gamut in analyzing data to search for patterns that could lead to better outcomes: from neonatal monitoring to neurological disease diagnosis and tracking, and from obesity programs to monitoring the development of symptoms in cystic fibrosis.
One of Wang’s projects is specifically tracking patients with sickle-cell anemia. “We have to develop ways to help kids manage this disease,” she says, along with devices that can monitor indicators such as hydration levels or a spike in blood pressure, indicating a potential attack, and provide warnings if the conditions indicate the child should be taken to an emergency room immediately. “We developed a mobile app, using a very simple wristband that can provide an early warning before attacks happen,” Wang explains. “We can monitor them and see a trend,” she continues, often before the young patients notice anything amiss.
In a clinical trial with 29 youngsters, she says, only one showed signs of an incipient attack, and “we caught that case well before [the child] had the attack.” Wang continues, “We try to educate the kids themselves” to interpret the signals their monitoring systems provide and be able to respond in ways they’ve been taught.
Another of Wang’s projects is working with neonatal or pediatric ICU patients “to figure out how to better find the best medications and doses.” In addition, data collected and monitored in these settings can lead to better predictions of cardiac failure. “The continual monitoring devices collect a huge amount of data. And in a clinical setting, where there’s very accurate controls, you can know exactly when a heart attack happens, and you can look for early warning signs,” Wang says.
Her monitoring systems are also being used in research to try to detect early warning signs of neurological developmental disorders. “The earlier you can catch these conditions, the more you can fix,” she explains. “This is fundamental research. We’re looking at genetic data, molecular data, and comparing that with clinical data.”
Similar data-mining approaches are being applied to a panoply of diseases, including cancer in its many forms. “The hope is to find a biomarker that can give an early warning,” Wang goes on. One approach to that is “developing new molecular diagnostics, using nanotechnology.”
But big data come with their own set of big issues to grapple with, Wang points out. First is data quality control. “The quality of the data is often not good,” she says, with large numbers of incompatible and often obscure data formats for different kinds of test results and records. She also analyzes the records to find patterns that can provide guidance to physicians making decisions about when to discharge a given patient, for example. Tracking large numbers of patients with similar conditions and physiological indicators can show just how often they were readmitted for follow-up treatment and/or given different lengths of treatment before discharge.
The amounts of data being generated by such products are prodigious, so the only way to tackle such analysis is through the heavy use of artificial intelligence techniques, Wang says. So a big part of her research is to try to evaluate the relative effectiveness of different artificial intelligence systems, including IBM’s Watson.
Younger Lives Call for Durable Devices
While pediatric biomedical engineering poses a variety of particular challenges, it also has its own advantages. For one thing, by and large, younger patients are healthier to begin with rather than beset with the collection of ailments that tend to accumulate with age. “Pediatric patients in general are healthier, so they have the ability to heal better,” Guldberg says. On the other hand, with a long life ahead, any mechanical implants face the need for extreme durability—or the opposite, assured dissolution—to avoid the need for repeated surgeries.
“Even though it’s a challenge with pediatric technology to get things commercialized,” Guldberg says, such advances can have a disproportionate impact nationally and globally because of the great number of healthy years these young patients have ahead. “If we look overall at our healthcare system, if you can make people healthier earlier on, it could have a huge impact.”



